Acute Rejection by Belatacept (Bela) Dosing Frequency: Results from a Phase II Study of Kidney Transplant Recipients.
1UCSF, San Francisco, CA
2University Hospital of Nantes, Nantes, France
3University Hôpital of Bicêtre, Le Kremlin-Bicêtre, France
4Medizinische Hochschule Hannover, Hannover, Germany
5University Hospital of Bellvitge, Bellvitge, Spain
6Bristol-Myers Squibb, Lawrenceville, NJ
7Emory University Transplant Center, Atlanta, GA.
Meeting: 2016 American Transplant Congress
Abstract number: D133
Keywords: Biopsy, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
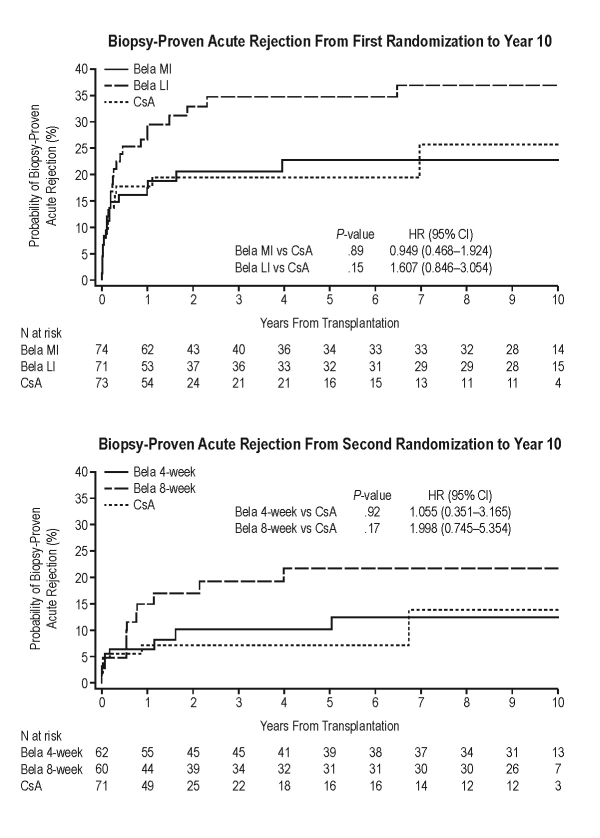
Background: At 1 year post transplant in IM103-100 (NCT00035555), bela-treated patients had similar acute rejection (AR) rates and significantly improved renal function vs cyclosporine A (CsA)-treated patients. This analysis examined biopsy-proven AR (BPAR) in CsA-treated and bela-treated patients at 10 years post-transplant.
Methods: Patients were first randomized to receive bela more intense (MI; n=74), bela less intense (LI; n=71), or CsA (n=73). At 3−6 months post-transplant, bela-treated patients underwent a second randomization to receive bela 5 mg/kg every 4 weeks (n=62) or 8 weeks (n=60). All randomized, transplanted patients were analyzed through 10 years post-transplant. BPAR was compared between regimens using Cox regression. BPAR was histologically confirmed at a central facility.
Results: Cumulative event rates for BPAR from first randomization to 10 years post-transplant were 23%, 37%, and 26% for bela MI, bela LI, and CsA, respectively. Hazard ratios (HRs) comparing BPAR did not differ statistically (Fig., top). BPAR from second randomization to 10 years post-transplant was most common in patients receiving bela 8-weekly; cumulative event rates for BPAR at 10 years post-transplant for bela 4-weekly, bela 8-weekly, and CsA were 11%, 22%, and 14%, respectively. One patient (randomized to bela 4-weekly) had grade IIB BPAR. No patient had grade III BPAR. Irrespective of dosing frequency, HRs comparing BPAR in bela-treated and CsA-treated patients did not differ significantly (Fig., bottom).
Conclusions: At 10 years post-transplant, rates of BPAR were similar between bela-treated and CsA-treated patients, with numerically higher rates of BPAR in patients receiving bela every 8 weeks vs every 4 weeks.
CITATION INFORMATION: Vincenti F, Blancho G, Durrbach A, Grannas G, Grinyó J, Meier-Kriesche U, Polinsky M, Zhao H, Larsen C. Acute Rejection by Belatacept (Bela) Dosing Frequency: Results from a Phase II Study of Kidney Transplant Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Vincenti F, Blancho G, Durrbach A, Grannas G, Grinyó J, Meier-Kriesche U, Polinsky M, Zhao H, Larsen C. Acute Rejection by Belatacept (Bela) Dosing Frequency: Results from a Phase II Study of Kidney Transplant Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/acute-rejection-by-belatacept-bela-dosing-frequency-results-from-a-phase-ii-study-of-kidney-transplant-recipients/. Accessed July 1, 2025.« Back to 2016 American Transplant Congress
