Acute Cellular Rejection in Liver Transplant Recipients Following Vaccination Against Covid-19
1Division of Gastroenterology, Hepatology and Nutrition, University of Minnesota, Minneapolis, MN, 2Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN
Meeting: 2022 American Transplant Congress
Abstract number: 525
Keywords: COVID-19, Liver transplantation, Rejection, Vaccination
Topic: Clinical Science » Liver » 54 - Liver: Immunosuppression and Rejection
Session Information
Session Name: Retransplantation and Other Complications
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:50pm-7:00pm
Presentation Time: 6:50pm-7:00pm
Location: Hynes Room 313
*Purpose: The coronavirus disease 2019 (COVID-19) global pandemic has seen the development of effective vaccines in record time. We report a series of five liver transplant (LT) recipients who developed acute cellular rejection (ACR) after receiving COVID-19 vaccinations.
*Methods: We performed a single-center, retrospective review of LT recipients who presented with biopsy-proven ACR after receiving a COVID-19 vaccination.
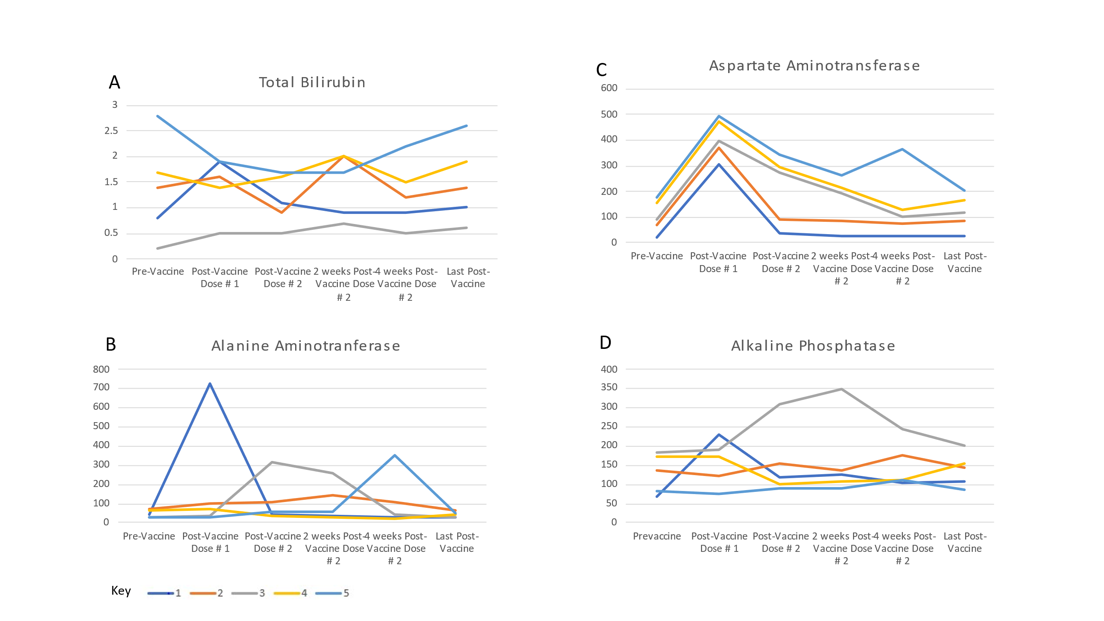
*Results: 603 LT recipients were fully vaccinated against COVID-19 at our center on 10/4/2021. Five (0.77%) patients developed elevated liver enzymes after COVID-19 vaccination without an identifiable cause and had a subsequent liver biopsy consistent with ACR: four (80%) patients were male and the median age was 54 years old. The indication for LT was cirrhosis secondary to non-alcoholic steatohepatitis in three (60%) and alcohol in two (40%) patients. The median time from LT to the first dose of COVID-19 vaccination was 19 months (range 7-26 months). Three (60%) patients had moderate (RAI= 5/9) ACR. All patients were treated with high-dose intravenous methylprednisolone for 3 days and had normalization of liver enzymes. No patients required rescue therapy with anti-thymocyte globulin or developed graft failure. All patients eventually completed their vaccination series.
*Conclusions: LT recipients may be at risk for developing ACR after the COVID-19 vaccination. Further study is required to better understand this relationship while closer monitoring following vaccination may be warranted in this patient population. Figure 1: Liver Enzymes of Five Patients Relative to Vaccine Doses
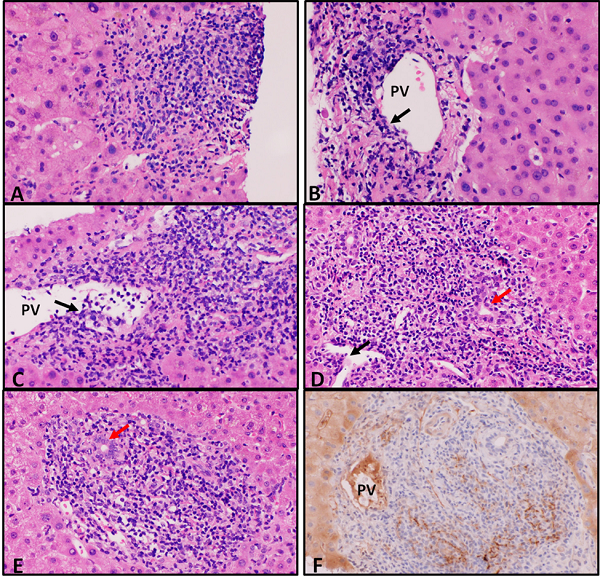
Figure 2: Liver Histopathology in Five Patients Showing Acute Cellular Rejection and C4d Staining in One Patient
| Pt. | Age, Gender and Etiology of liver disease | IS Regimen | Time since LT (days) | Last Pre-Vaccine TB/AST/ALT/ALP | Peak Post-Vaccine TB/AST/ALT/ALP | Liver Biopsy (RAI=n/9) | Treatment and Outcomes |
| 1 | 52, M Alcohol | MMF 750mg BID + PRED 5mg daily | 827 | 0.8/19/42/67 | 2.8/22/25/82 | ACR (5/9) | METHYLPRED 1g IV x3 days, Oral PRED taper, Added EVE. Improved |
| 2 | 71, M Alcohol | TAC (goal 7-10) + MPA 720mg BID | 1029 | 1.4/50/72/136 | 2.4/61/128/128 | ACR (3/9) | METHYLPRED 500mg IV x3 days, Oral PRED taper.Improved, Repeat biopsy on day 890 since LT showed mild portal and lobular inflammation with pseudo ground-glass inclusions |
| 3 | 50, M
NASH
|
TAC (goal 7-10) + MPA 720mg BID | 438 | 0.2/20/25/182 | 0.7/184/318/349 | Immune-med (2-3/9) | Improved, Repeat biopsy on day 890 since LT showed mild portal and lobular inflammation with pseudo ground-glass inclusions.Improved |
| 4 | 61, F NASH/A1AT/Iron Overload | CSA (goal 80-120) | 778 | 1.7/68/65/172 | 2.0/75/72/180 | ACR (5/9), focal C4d + | METHYLPRED 1g IVx3 days, Oral PRED taper, Increased CSA goal, Added MMF.Improved |
| 5 | 54, M NASH | MMF 750mg BID + PRED 5mg daily | 906 | 2.8/22/25/82 | 2.2/235/354/119 | ACR (5/9) | METHYLPRED 500mg IV x3 days, Oral PRED taper, Added TAC.Improved |
To cite this abstract in AMA style:
Sarwar R, Adeyi O, Lim N. Acute Cellular Rejection in Liver Transplant Recipients Following Vaccination Against Covid-19 [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/acute-cellular-rejection-in-liver-transplant-recipients-following-vaccination-against-covid-19/. Accessed February 26, 2026.« Back to 2022 American Transplant Congress