A Six-Month, Prospective, Single-Center, Pilot Study to Determine the Pharmacokinetics and Effectiveness of Immunosuppressant Regimens in Liver Transplantation Patients Receiving Twice-Daily Tacrolimus and Everolimus (TAC + EVR BID) Regimen Converted to Once-Daily Tacrolimus and Everolimus (TAC + EVR QD) Regimen – A Priliminary Report
Division of Liver and Transplantation Surgery, Chang-Gung Memorial Hospital, Chang-Gung University College of Medicine, Taoyuan, Taiwan.
Meeting: 2018 American Transplant Congress
Abstract number: C214
Keywords: Efficacy, Immunosuppression, Liver, Pharmacokinetics
Session Information
Session Name: Poster Session C: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Adherence to immunosuppressant regimen is crucial for graft survival and usually inversely related to the dose frequency. Similar efficacy and safety profile has been approved for once-daily(QD) TAC. EVR QD dosing has also shown the comparable result for renal transplant. We examined the pharmacokinetics(PK) and efficacy of liver transplant recipients receiving twice-daily TAC+EVR regimen(BID) and then being shift to QD dosing.
The study enrolled ten adult patients who received de novo liver transplant within 6 months and used TAC+EVR BID regimen and eGFR≥30 ml/min/1.73 m2. The primary end point was the PK study of TAC+EVR under BID and QD dosing. The blood sample of PK study were obtained at pre-morning dosing (0 hr) and 1,2,3,4,5,6,8,10,12,16,24 hrs after drug administration. TAC+EVR was shift to QD dosing by 1:1 proportion. PK study of QD dosing was performed after 2 weeks. 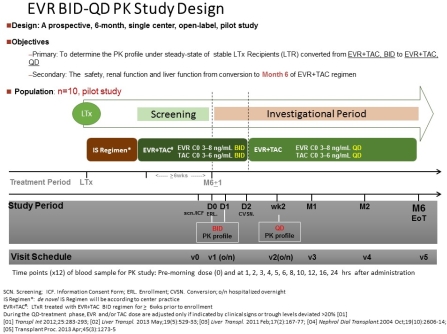 The secondary end points were treatment failure, report of adverse events(AE), and measurement of eGFR. The drug level of TAC and EVR level BID and QD dosing in first 2 patients was similar.
The secondary end points were treatment failure, report of adverse events(AE), and measurement of eGFR. The drug level of TAC and EVR level BID and QD dosing in first 2 patients was similar.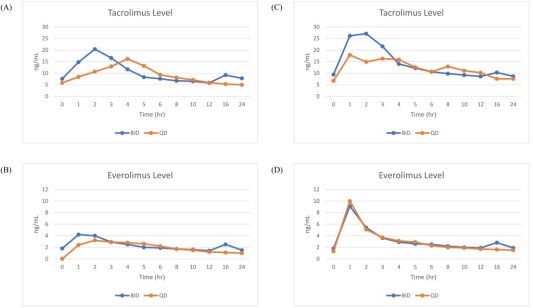 The graft function and eGFR were comparable
The graft function and eGFR were comparable
| AST(U/L) | ALT(U/L) | Total Bilirubin(mg/dL) | INR | eGFR (ml/min/1.73m2) | |
| P't 1 (BID/QD) | 21/22 | 12/12 | 0.6/0.6 | 1.0/1.0 | >60/57 |
| P't 2 (BID/QD) | 22/23 | 14/12 | 1.0/0.5 | 1.1/1.0 | >60/>60 |
Our preliminary data suggested that shift TAC+EVR from BID to QD dosing could be considered.
CITATION INFORMATION: Wu T-.H., Cheng C-.H., Wang Y-.C., Lee C-.F., Wu T-.J., Chou H., Chan K-.M., Lee W-.C. A Six-Month, Prospective, Single-Center, Pilot Study to Determine the Pharmacokinetics and Effectiveness of Immunosuppressant Regimens in Liver Transplantation Patients Receiving Twice-Daily Tacrolimus and Everolimus (TAC + EVR BID) Regimen Converted to Once-Daily Tacrolimus and Everolimus (TAC + EVR QD) Regimen – A Priliminary Report Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Wu T-H, Cheng C-H, Wang Y-C, Lee C-F, Wu T-J, Chou H, Chan K-M, Lee W-C. A Six-Month, Prospective, Single-Center, Pilot Study to Determine the Pharmacokinetics and Effectiveness of Immunosuppressant Regimens in Liver Transplantation Patients Receiving Twice-Daily Tacrolimus and Everolimus (TAC + EVR BID) Regimen Converted to Once-Daily Tacrolimus and Everolimus (TAC + EVR QD) Regimen – A Priliminary Report [abstract]. https://atcmeetingabstracts.com/abstract/a-six-month-prospective-single-center-pilot-study-to-determine-the-pharmacokinetics-and-effectiveness-of-immunosuppressant-regimens-in-liver-transplantation-patients-receiving-twice-daily-tacrolimu/. Accessed February 19, 2026.« Back to 2018 American Transplant Congress
