A Randomized Controlled Clinical Trial of Thymoglobulin® and Extended Delay of Calcineurin Inhibitor Therapy for Renal Protection after Liver Transplantation: A Multicenter Study
1The Cleveland Clinic, Cleveland, OH, 2University of Cincinnati, Cincinnati, OH, 3The Cleveland Clinic, Weston, FL, 4Medical College of Wisconsin, Milwaukee, WI
Meeting: 2019 American Transplant Congress
Abstract number: 550
Keywords: Immunosuppression, Induction therapy, Liver transplantation, Renal function
Session Information
Session Name: Concurrent Session: Liver - Kidney Issues in Liver Transplantation
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 302
*Purpose: Thymoglobulin® (r-ATG) has been used as induction therapy in liver transplantation (LT). No prospective, randomized, controlled, trial (RCT) has been performed to evaluate effect of r-ATG induction and delayed initiation of CNI on long-term renal function after LT
*Methods: 110 patients were randomized to r-ATG induction with delayed initiation of CNI for 10 days after LT, and standard CNI group, at 4 transplant programs. The eGFR and delta GFR were measured at 1, 3, 6, 9, and 12-month milestones for analysis
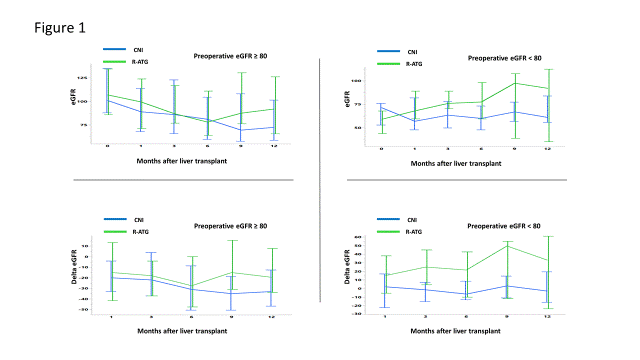
*Results: The median age, MELD score, baseline creatinine, baseline eGFR, and gender were similar between the two groups (all P>0.05). The median baseline, post-1, -3, -6, -9 and -12 month eGFR in CNI vs r-ATG groups were 88.0 vs. 91.6 (0.18), 82.0 vs. 93.5 (P=0.045), 75.0 vs. 86.0 (P=0.046), 74.0 vs. 79.0 (P=0.15), 69.5 vs. 97.0 (P=0.01), and 73.0 vs.90.0 (P=0.08). The median delta eGFR at 12 months after transplant was better in r-ATG group although it did not reach statistically significant (-21 vs -12, P=0.23). The time-weighted average of CNI levels were similar between two groups (CNI group; 7.2ng/mL r-ATG group 7.5ng/mL P=0.60). The chronological changes of median and interquartile ranges of eGFR and delta eGFR according to pre-transplant GFR (≥ or <80) were shown in Figure 1, and Table 1. The protective influence of r-ATG was more pronounced (approaching significance), in patients with lower initial GFR at LT
*Conclusions: Early initiation of CNI has shown to affect long-term renal function after LT. Induction with r-ATG and delayed initiation of CNI seems to be protective of long-term renal function in LT. This effect is seen at every milestone of follow up but more pronounced in patients with initial degree of renal dysfunction, especially in the era of LT in high-MELD recipients
| Baseline | 88 (75-111) | 91 (80-132) | 0.18 |
| 1 month | 82 (54-102) | 93 (68-124) | 0.045 |
| 3 months | 75 (57-110) | 86 (76-117) | 0.046 |
| 6 months | 74 (58-98) | 79 (64-107) | 0.15 |
| 9 months | 69 (62-88) | 97 (77-129) | 0.01 |
| 12 months | 73 (60-87) | 90 (61-122) | 0.08 |
To cite this abstract in AMA style:
Coromina L, Shah S, Xervos B, Zimmerman M, Sasaki K, Diago T, Hashimoto K, Aucejo F, Fujiki m, Block-Beach H, Patterson A, Miller C, Quintini C, Eghtesad B. A Randomized Controlled Clinical Trial of Thymoglobulin® and Extended Delay of Calcineurin Inhibitor Therapy for Renal Protection after Liver Transplantation: A Multicenter Study [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/a-randomized-controlled-clinical-trial-of-thymoglobulin-and-extended-delay-of-calcineurin-inhibitor-therapy-for-renal-protection-after-liver-transplantation-a-multicenter-study/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress