A Prospective Randomized Multicenter Trial (B E S T Trial) of Belatacept-Based C N I- and Corticosteroid-Free Immunosuppression: Final Two Year Results
1University of Wisconsin, Madison, WI, 2University of Cincinnati, Cincinnati, OH, 3Tampa General, Tampa, FL, 4University of Colorado School of Medicine, Denver, CO, 5University of Minnesota, Minneapolis, MN, 6University of Illinois, Chicago, Chicago, IL, 7University of Cincinnati, Cincinnati, WI
Meeting: 2019 American Transplant Congress
Abstract number: 309
Keywords: Graft function, Immunosuppression, Induction therapy, Kidney transplantation
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Novel Regimens and Drug Minimization II
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Veterans Auditorium
*Purpose: The BEST Trial (Belatacept-based Early Steroid Withdrawal Trial) is a prospective, randomized, multi-center trial designed to compare two belatacept (BELA)-based calcineurin inhibitor-free (CNI), early corticosteroid withdrawal (ESW) regimens with a standard of care (SOC) tacrolimus (TAC)-based ESW regimen. This report presents final two-year results of this trial.
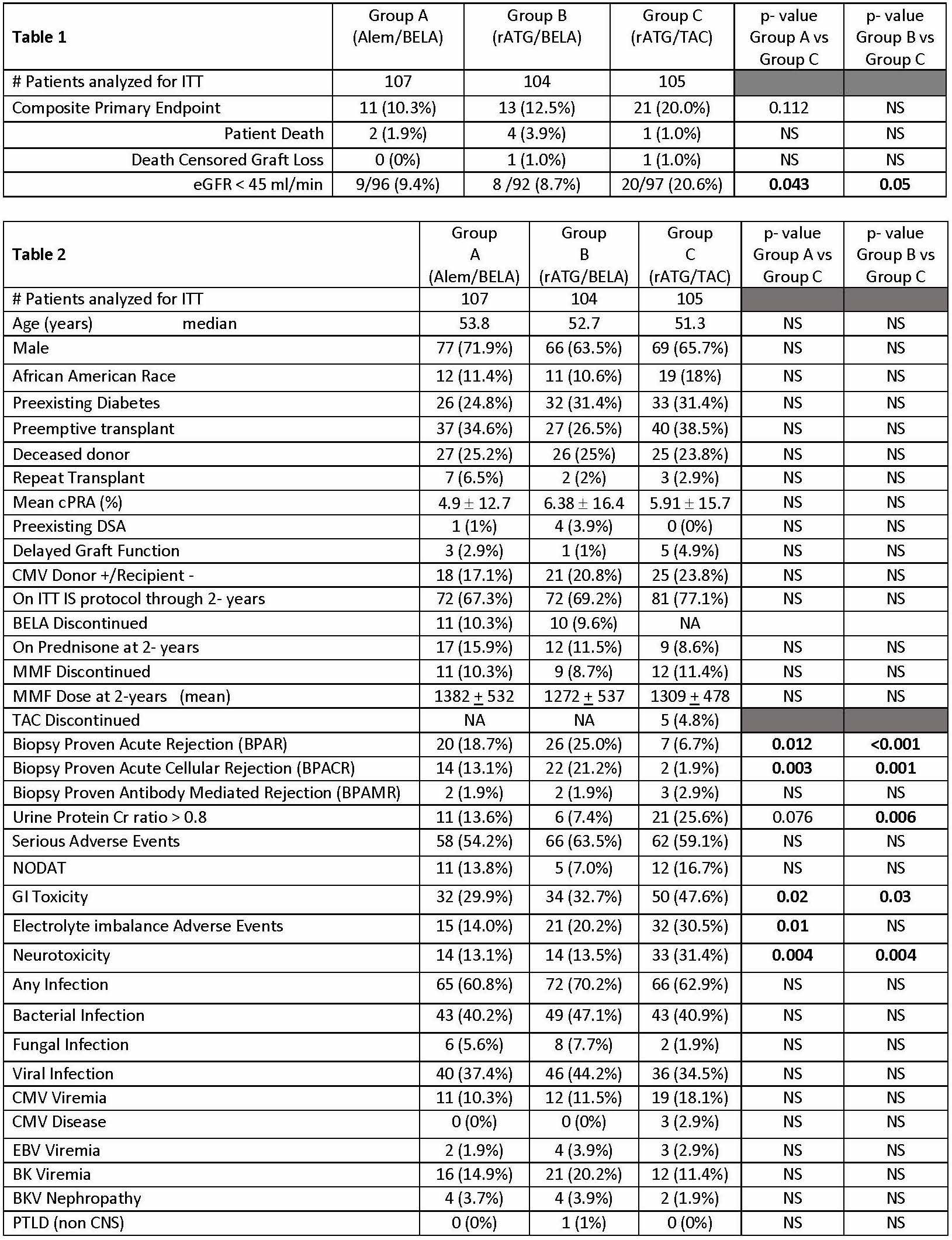
*Methods: This study was conducted under an FDA IND and IRB approval at each of eight sites. Adult kidney transplant (KTx) recipients of living and deceased donor allograft were eligible for enrollment. All patients received mycophenolate therapy and a five-day steroid taper. 316 patients were randomized to three treatment groups: alemtuzumab + BELA (Group A), rATG + BELA (Group B), and rATG + TAC (Group C). Primary endpoint was a composite of: patient death or graft loss or estimated GFR < 45ml/min/m2 and the components are presented as 2-year actuarial rates in Table 1.
*Results: Enrollment was completed on 12/15/16. Intent to treat (ITT) analyses on patients with 2 year follow-up as of 11/1/18 are presented in Table 2.
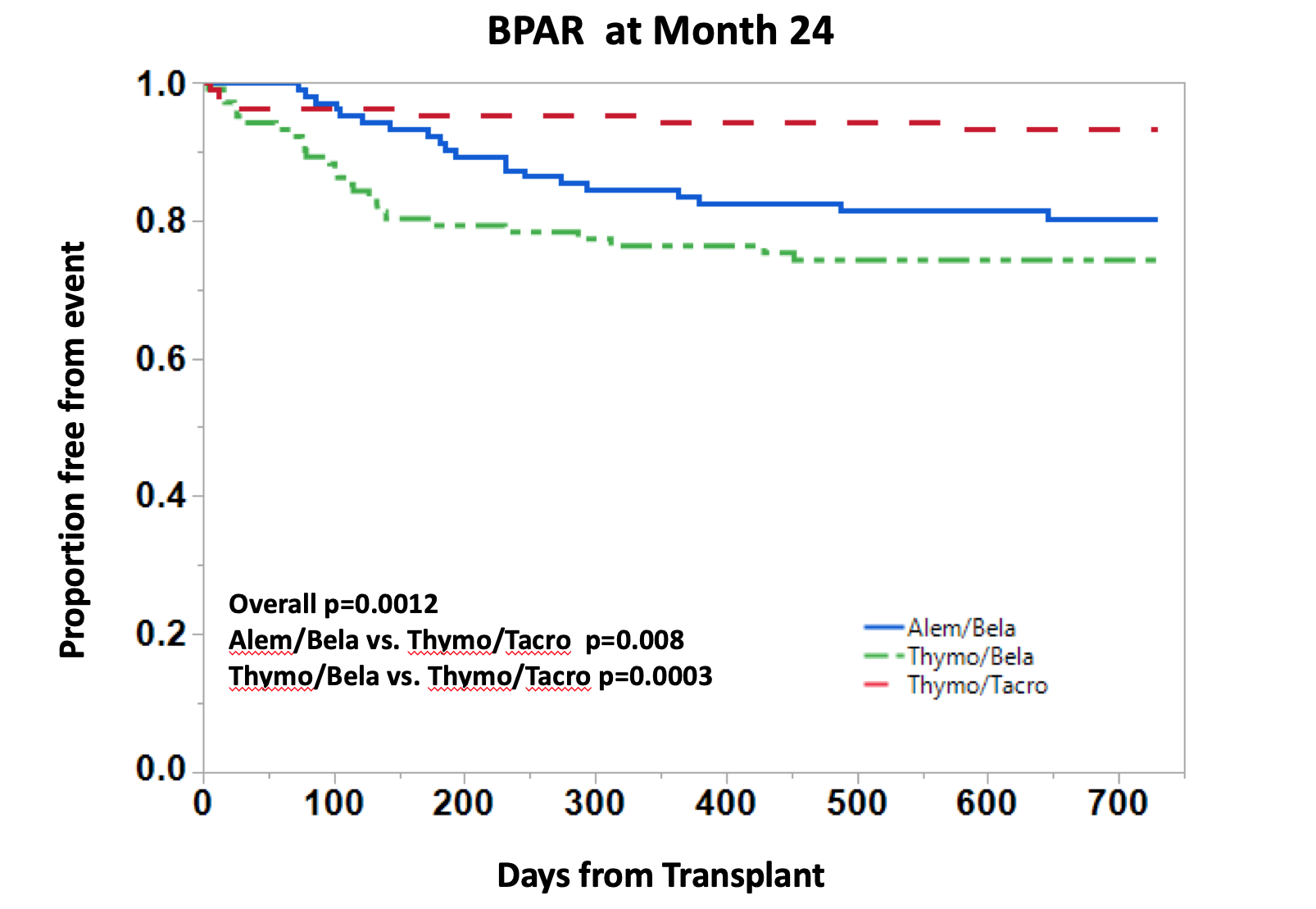
*Conclusions: The primary endpoint was similar among all groups, however, there were differences in secondary endpoints. Regarding quality of renal function, the proportion of patients with eGFR <45ml/min was significantly higher in the TAC group. However, rejection rates were significantly higher in the BELA groups, and approximated 20-25% over 2-years. Tolerability (GI toxicity, neurotoxicity, and metabolic adverse events) was significantly improved in BELA treated patients compared to TAC. This CNI- and steroid-free IS protocol is a promising step forward in minimizing toxicities and improving renal allograft function. Longer-term observations will need to be continued.
To cite this abstract in AMA style:
Kaufman DB, Shields AR, Leone JP, Wiseman A, Matas AJ, West-Thielke P, Lipscomb J, King E, Alloway RR. A Prospective Randomized Multicenter Trial (B E S T Trial) of Belatacept-Based C N I- and Corticosteroid-Free Immunosuppression: Final Two Year Results [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/a-prospective-randomized-multicenter-trial-b-e-s-t-trial-of-belatacept-based-c-n-i-and-corticosteroid-free-immunosuppression-final-two-year-results/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress