A Prospective Iterative Trial of Carfizomib-Based Desensitization Trial: Initial Comparative Observations.
1U of Cincinnati, Cincinnati
2Christ Hospital, Cincinnati
Meeting: 2017 American Transplant Congress
Abstract number: 105
Keywords: Antibodies, B cells, HLA antibodies, Kidney transplantation
Session Information
Session Name: Concurrent Session: Clinical Science: Kidney Immunosuppression: Desensitization
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: E354b
Carfilzomib (CFZ) is a 2nd generation proteasome inhibitor different form bortezomib in that it irreversibly disables 20s and 26s proteasomes.Results from the first 2 phases of a prospective iterative CFZ-based desensitization (DS) trial are presented.
Methods: All patients received CFZ (12 escalating doses up to 36mg/m2). Phase 2 added 6 weekly plasmapheresis treatments prior to CFZ. Primary and secondary endpoints were safety and reduction in iAb. Antibody specificities were defined by single antigen beads. cPRA was calculated using a precision calculator based on the OPTN cPRA formula. Enabled PRA was determined by entering in the calculator specificities that were lowered below predefined MFI thresholds. Donors required to match (DRTM) were defined as (1/1-cPRA) and fold decrease in DRTM as (DRTM pre-DS/DRTM post-DS).
Results: 6 patients were enrolled in phase 1 and 4 in phase 2. Treatment was well tolerated. Grade 4 toxicities were not observed. 3 patients had grade 1/2 thrombocytopenia, 2 had paresthesia/neuropathy while 2 had grade 3 toxicities (transaminitis and neutropenia). iAb was reduced by ≥ 85% in 50% of patients at day 53. One patient had a significant increase in class II iAb due to an upper respiratory tract infection. # of HLA Ab specificities were substantially reduced. Tables 1a and 1b summarize iAb and cPRA results. Fig. 1 summarizes # of HLA specificities. Class II antibodies were eliminated in 1 patient at at 1:8 titer.
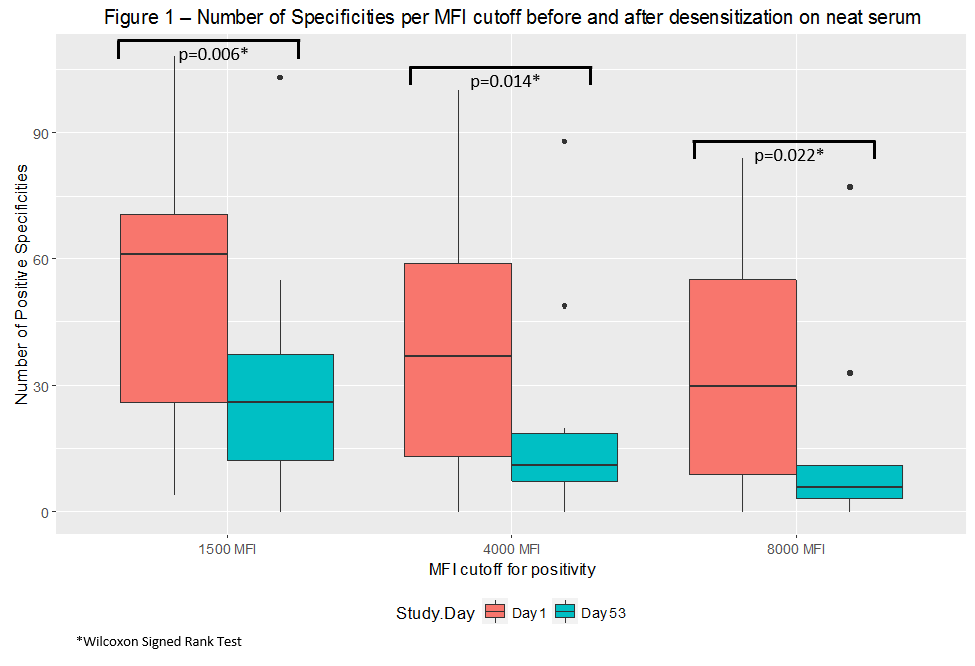 Conclusion: Escalating doses of CFZ were well tolerated and led to important reductions in iAb and # of HLA specificities in highly sensitized patients. Addition of weekly plasmapheresis to CFZ dosing did not appear to have a robust impact on iAb response. Future groups will evaluate additive effect of rituximab.
Conclusion: Escalating doses of CFZ were well tolerated and led to important reductions in iAb and # of HLA specificities in highly sensitized patients. Addition of weekly plasmapheresis to CFZ dosing did not appear to have a robust impact on iAb response. Future groups will evaluate additive effect of rituximab.
CITATION INFORMATION: Tremblay S, Shields A, Alloway R, Brailey P, Leino A, Lichvar A, Abu Jawdeh B, Driscoll J, Girnita A, Woodle E. A Prospective Iterative Trial of Carfizomib-Based Desensitization Trial: Initial Comparative Observations. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Tremblay S, Shields A, Alloway R, Brailey P, Leino A, Lichvar A, Jawdeh BAbu, Driscoll J, Girnita A, Woodle E. A Prospective Iterative Trial of Carfizomib-Based Desensitization Trial: Initial Comparative Observations. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-prospective-iterative-trial-of-carfizomib-based-desensitization-trial-initial-comparative-observations/. Accessed February 28, 2026.« Back to 2017 American Transplant Congress
