A Prospective Carfilzomib-Based Desensitization Trial: Phase 1 Results.
1U Of Cincinnati, Cincinnati
2Mayo Clinic, Phoenix.
Meeting: 2016 American Transplant Congress
Abstract number: 318
Keywords: Alloantibodies, B cells, Kidney, Sensitization
Session Information
Session Name: Concurrent Session: Kidney: Desensitization
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 304
Carfilzomib (CFZ) is mechanistically distinct from bortezomib as it is an irreversible PI. Results from phase 1 of a CFZ desensitization (DS) trial are presented
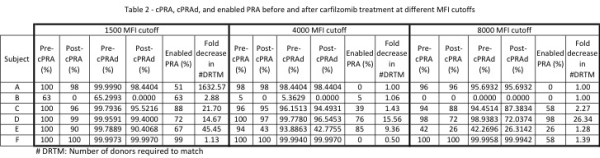
Methods: 6 pts each received 12 escalating CFZ doses (20,27,36mg/m2) followed by 3 plasmapheresis. Primary efficacy endpoint is immunodominant antibody (iAb) reduction. HLA antibody (Ab) specificities were defined by single antigen beads (SAB). cPRA was calculated with the OPTN cPRA calculator. Enabled PRA was calculated by entering HLA specificities reduced by DS below a defined MFI level (1500, 4000 of 8000 MFI). High-precision PRA (cPRAd) values were derived using a cPRAd calculator that provided PRA values to several decimals. Number of donors required to match (DRTM) was calculated by: 1/(1-pPRA) and fold decrease in DRTM was defined as (DRTM pre-treatment)/(DRTM post-treatment).
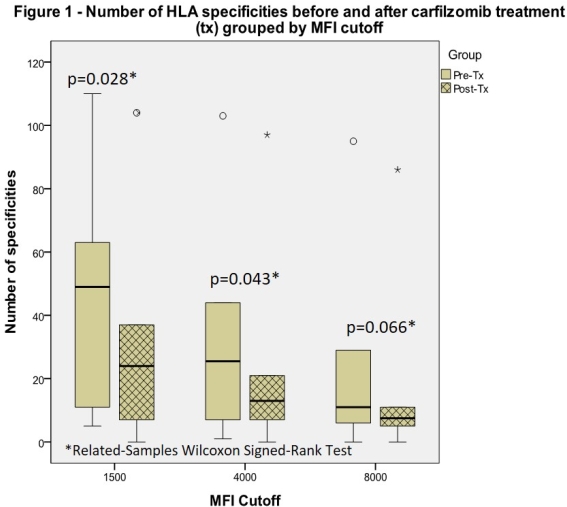
Results: 6 patients completed Phase 1;5/6 patients had a cPRA > 96%. iAb decreases averaged 81.6% at day 53 and 83% had iAb decreases > 80%. iAb values at several time points in a single patient during CFZ treatment alone revealed an iAb decrease of 71%. # of HLA Ab specificities were substantially reduced. Tables 1 and 2 summarize iAb and cPRA results. Figure 1 summarizes # of HLA specificities. Toxicities included 2 grade 1 transaminase elevations and a grade 1 peripheral neuropathy that resolved in 7 days.
| ID | iDSA specificity | Pre-treatment iAb Titer | pre-treatment cPRA (%) |
D0 iAb MFI |
D53 iAb MFI |
Reduction iAb (%) |
| A | DQ7.2,DQ9,DQ7.5 | Neat | 98 | 10,330 | 417 | 96 |
| B | A31 | Neat | 58 | 4022 | 33 | 99.2 |
| C | B12 creg | 1:32 | 100 | 8192 | 1494 | 81.8 |
| D | A29,A36 | 1:64 | 100 | 3934 | 558 | 85.8 |
| E | B7 creg | 1:8 | 97 | 5980 | 3362 | 42.9 |
| F | A2 | 1:1024 | 100 | 12910 | 2061 | 84 |
Conclusions: CFZ monotherapy provides substantial iAb reductions with minimal toxicities.
CITATION INFORMATION: Tremblay S, Shields A, Alloway R, Brailey P, Abu Jawdeh B, Latif T, Driscoll J, Pando M, Girnita A, Woodle E. A Prospective Carfilzomib-Based Desensitization Trial: Phase 1 Results. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Tremblay S, Shields A, Alloway R, Brailey P, Jawdeh BAbu, Latif T, Driscoll J, Pando M, Girnita A, Woodle E. A Prospective Carfilzomib-Based Desensitization Trial: Phase 1 Results. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/a-prospective-carfilzomib-based-desensitization-trial-phase-1-results/. Accessed February 22, 2026.« Back to 2016 American Transplant Congress
