A Pathway for Establishing Etiology of Kidney Failure: An Academic Transplant Center’s Experience with a Broad Kidney Gene Panel
1Loyola University Medical Center, Maywood, IL, 2Natera, Inc., Austin, TX
Meeting: 2022 American Transplant Congress
Abstract number: 505
Keywords: Gene polymorphism, Kidney, Recurrence
Topic: Clinical Science » Kidney » 49 - Recurrent Kidney Disease & Genetics
Session Information
Session Name: Recurrent Kidney Disease & Genetics
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:30pm-6:40pm
Presentation Time: 6:30pm-6:40pm
Location: Hynes Room 310
*Purpose: Assessing genetic causes of kidney failure can inform pre-transplant decision making and may improve post-transplant outcomes. Here we describe an academic transplant center’s experience implementing genetic testing with a broad next-generation sequencing (NGS)-based gene panel in evaluating patients at high-risk of monogenic kidney disease and/or APOL1-related disease pre- and post-transplant.
*Methods: Kidney transplant (KT) patients were tested via a 382 or 385-gene panel related to kidney disorders if they (i) were age <50 years with an unknown cause of kidney disease, (ii) had kidney disease not explained by comorbidities, (iii) had biopsy-proven focal segmental glomerulosclerosis (FSGS) or nephrotic-range proteinuria, (iv) had a family history of kidney disease without a known etiology, or (v) for identification of clinical disease subcategory for selection of related living donor selection. Pathogenic and/or likely pathogenic variants associated with X-linked (XL), autosomal dominant (AD), autosomal recessive (AR), or complex (APOL1-related) disease were considered positive.
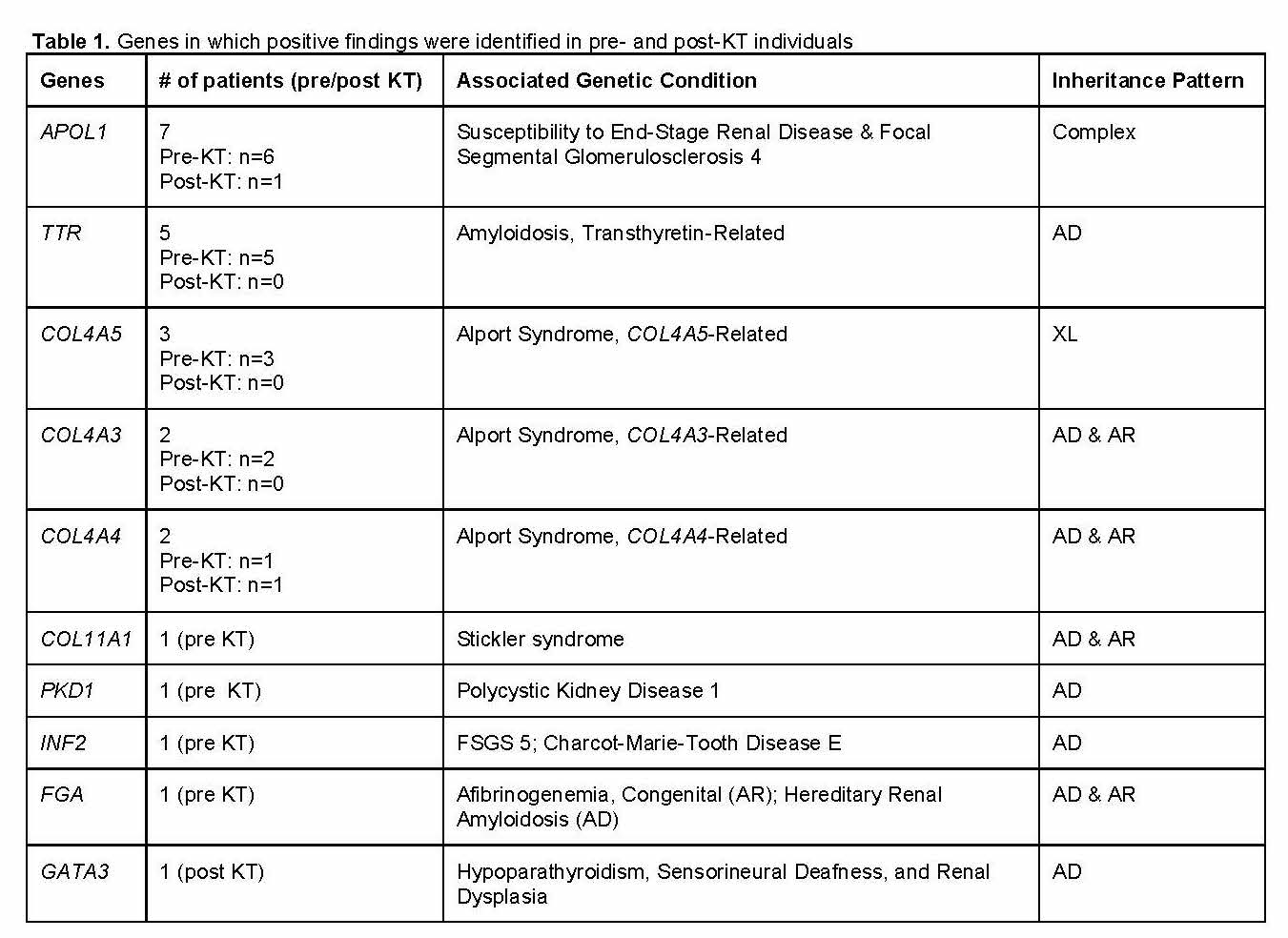
*Results: Genetic testing was performed on 68 patients (pre-KT: n=59; post-KT: n=9) aged 20-70 years (median: 36). Positive findings were identified in 29% (20/68) patients (pre-KT: n=17; post-KT: n=3) in 10 genes (Table 1). Four pre-KT patients had dual positive findings in TTR and APOL1 (n=2 each: G1/G1 and G1/G2). Fourteen pre-KT individuals subsequently underwent KT, 3 of whom had positive findings resulting in reclassification of presumed FSGS as collagen-based disorders (COL4A5: n=2; COL11A1: n=1) indicating a low likelihood of recurrence post-KT. Four other pre-KT individuals with presumed FSGS were reclassified to collagen-based disorders (COL4A3, COL4A4, COL4A5) and as APOL1 (G1/G1) with TTR. Positive findings in post-KT patients resulted in reduced suspicion for risk of recurrent FSGS (for an APOL1 G1/G2 finding), counseling of a related living donor (for which a positive COL4A4 finding was identified in the recipient), and reclassification of disease for 1 patient with suspected Alport syndrome to renal dysplasia (for a positive finding in GATA3).
*Conclusions: Use of defined inclusion criteria and a broad kidney gene panel resulted in a high diagnostic yield of genetic kidney diseases. Identification of pre- and post-KT patients at increased risk of monogenic kidney disease has implications for recurrence risk, at-risk relatives, and targeted treatments, which can improve outcomes post-KT.
To cite this abstract in AMA style:
Sodhi R, Brossart K, Desai A, Arwindekar DJ, Tabriziani H, Akkina S. A Pathway for Establishing Etiology of Kidney Failure: An Academic Transplant Center’s Experience with a Broad Kidney Gene Panel [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/a-pathway-for-establishing-etiology-of-kidney-failure-an-academic-transplant-centers-experience-with-a-broad-kidney-gene-panel/. Accessed July 2, 2025.« Back to 2022 American Transplant Congress