A Novel Formula to Determine Optimal Immunosuppression in Renal Transplant Recipients Treated with Tacrolimus and Mycophenolate Mofetil: Towards Individualized Immunosuppression
1SUNY, Upstate Medical University, Syracuse, NY, 2Cukurova University, Adana, Turkey, 3SUNY, Downstate Medical University, New York, NY
Meeting: 2020 American Transplant Congress
Abstract number: B-311
Keywords: Area-under-curve (AUC), Dosage, Kidney transplantation, Mycophenolate mofetil
Session Information
Session Name: Poster Session B: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Tacrolimus (TAC) and mycophenolic acid (MPA) remain the most commonly used drug combination for kidney transplant immunosuppression. Adequate drug dosing is challenging due to the lack of a reliable method of assessing optimal drug exposure. Therapeutic drug monitoring (TDM) for TAC is well established. However, the value of TDM for MPA remains unclear. We evaluated the value of combining time integrated trough TAC levels and MPA exposure in predicting clinical outcomes.
*Methods: This retrospective single center study included 110 renal transplant recipients having 113 separate events treated with TAC and mycophenolate mofetil (MMF). MPA and MPA-G AUC were measured by limited sampling strategy within the first 6 months post-transplant. Outcomes over the first year were collected and analyzed.
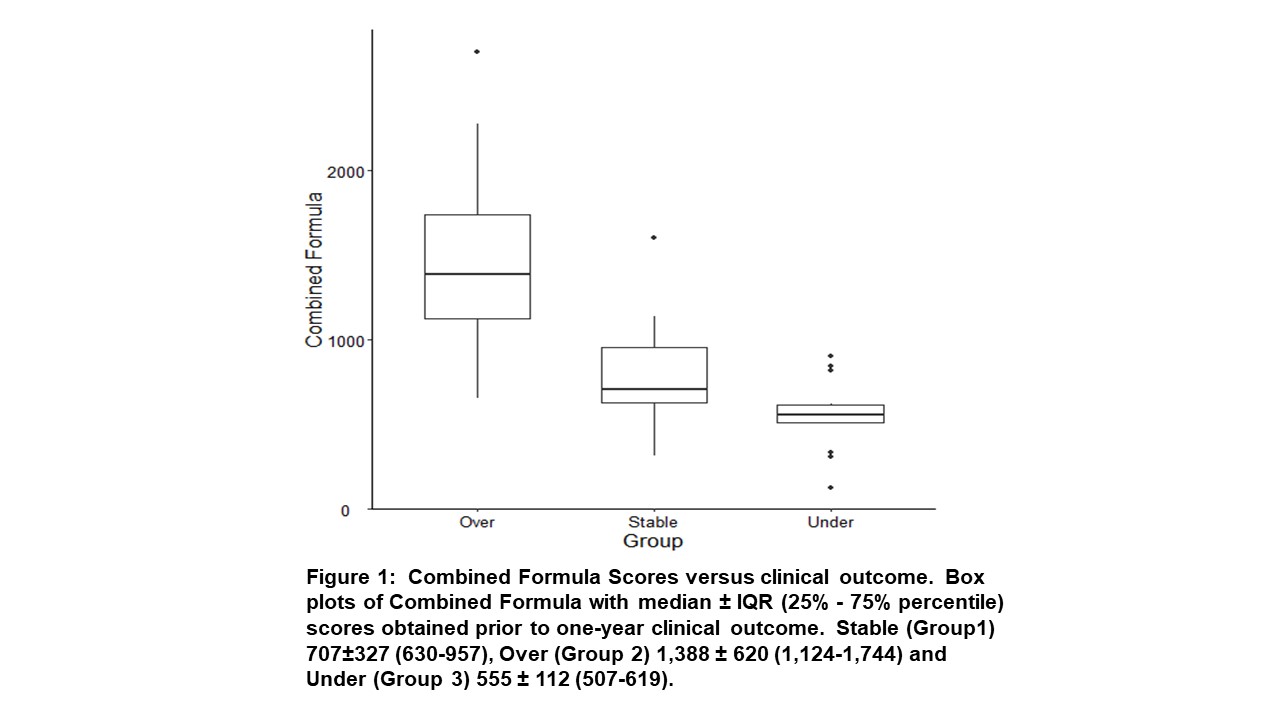
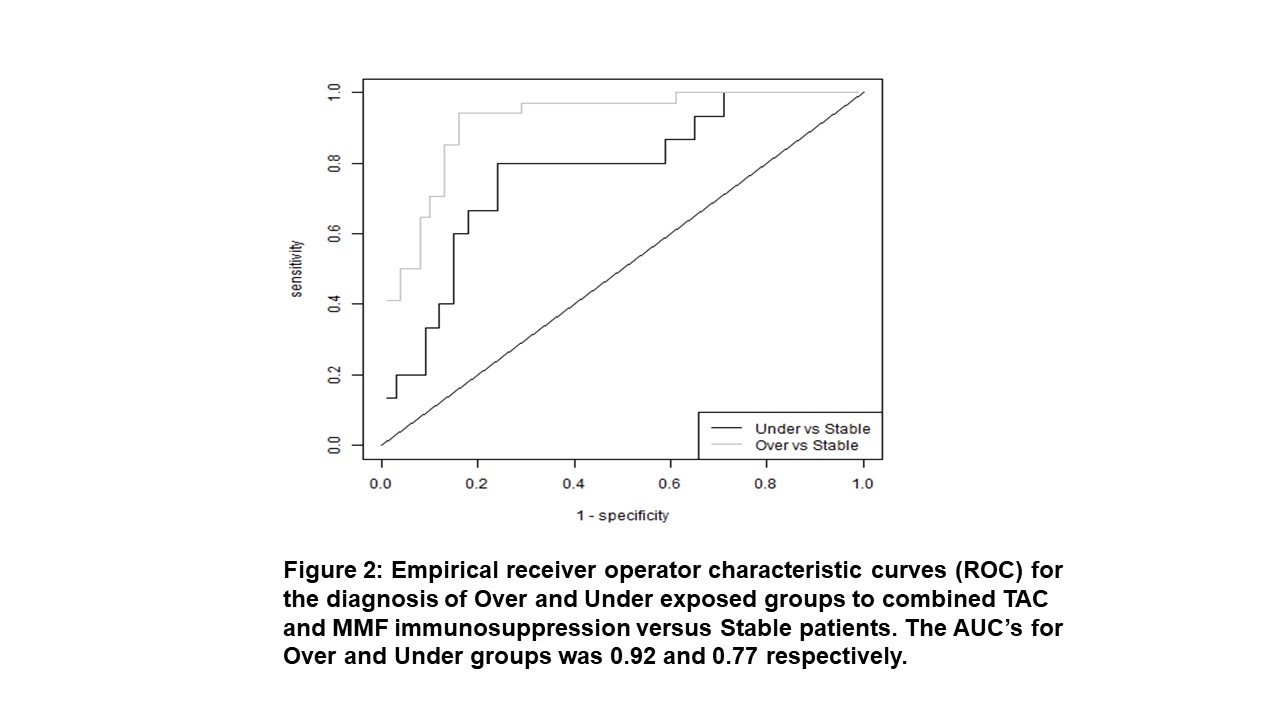
*Results: Patients were classified into 3 groups: Stable (n=34) having an uncomplicated course, Over drug exposed (n=64) experiencing viral infections or drug toxicity and Under drug exposed (n=15) developing rejection or dnDSA. The groups had similar demographics . Although TAC, MPA and MPA-G exposure differed between groups the individual values were either not predictive or had exceedingly narrow ranges to be clinically useful. Combining the TAC, MPA and MPA-G exposure into one calculation the Combined Formula: TAC TDM X (MPA AUC + MPA-G AUC/10) provided the greatest separation between groups and identifies both over and under drug exposed patients (Figure 1). The ROC curves for the Combined Formula had AUC values of 0.77 and 0.92 for under and over drug exposure respectively. The sensitivity and specificity of a Combined Formula score of 1,071 for over exposure is 0.94 (95% CI 0.56-0.83) and 0.84 (95% CI 0.69-0.89) respectively. For under exposure a score of 625 has a sensitivity and specificity of 0.76 (95% CI 0.53-0.93) and 0.80 (95% CI 0.41-0.70) (Figure 2).
*Conclusions: This practical and more reliable method to assess overall tacrolimus and MPA effect may facilitate individualized immunosuppression.
To cite this abstract in AMA style:
Pankewycz O, Laftavi M, Onan E, Rucker D, Wang D, Bhuta K, Gruessner R, Gruessner A. A Novel Formula to Determine Optimal Immunosuppression in Renal Transplant Recipients Treated with Tacrolimus and Mycophenolate Mofetil: Towards Individualized Immunosuppression [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/a-novel-formula-to-determine-optimal-immunosuppression-in-renal-transplant-recipients-treated-with-tacrolimus-and-mycophenolate-mofetil-towards-individualized-immunosuppression/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress