A Novel Combined Formula to Direct Optimal Immunosuppressive Drug Exposure in Renal Transplant Recipients
1Upstate Medical University, Syracuse, NY
2Cukurova University, Adana, Turkey
3Downstate Medical University, New York, NY.
Meeting: 2018 American Transplant Congress
Abstract number: C89
Keywords: Area-under-curve (AUC), Efficacy, Monitoring, Outcome
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: The most widely used maintenance immunosuppressive combination after kidney transplantation is tacrolimus (TAC) and mycophenolic acid (MPA). The utility of therapeutic drug monitoring for TAC is well established. In contrast, the routine application of monitoring MPA exposure has been limited due to its questionable clinical utility. However, the relevance MPA area under curve (AUC) has been studied without considering CNI exposure. In this study, we combine tacrolimus trough levels and the MPA (AUC) together with its metabolite MPA-G in a simple “Combined Formula” that best correlates with clinical outcomes in renal transplant recipients (RTR).
Methods: Patient charts were reviewed from 8/16 to 11/17. MPA and MPA-G AUC was calculated by limited sampling strategy (LSS). TAC exposure was recorded as the time averaged TAC level for the month prior to the MPA AUC in stable RTR or prior to a defining clinical event such as rejection, drug toxicity or infection. The “Combined Formula” used to determine total drug exposure was: TAC X (MPA AUC + MPA-G AUC/10). All RTR had similar demographics and received induction therapy, steroids, TAC and mycophenolate mofetil (MMF). Clinical outcomes were defined as: (1) Stable (SBL) no rejection, de novo DSA (dnDSA), viral infection or drug toxicity (2) over immunosuppression (OVR) viral illness or drug toxicity requiring MMF dose reduction or (3) under immunosuppression (UND) biopsy proven rejection or dnDSA.
Results: A total of 74 RTR were studied. Of these, 37 were SBL, 29 were OVR and 8 were in the UND group. By combining TAC and MPA+MPA-G/10 AUC levels, only the Combined Formula differentiated amongst all three groups and provided clinically meaningful separations. * p = 0.02 v SBL and <0.001 v OVR, + p = <0.001 v SBL 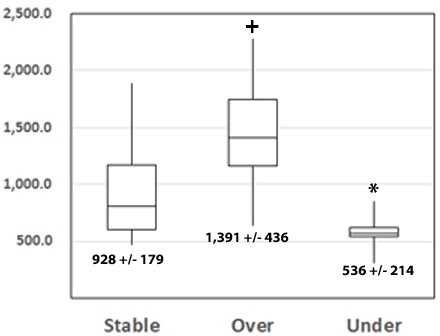
Conclusions: This useful and straightforward method to measure overall immunosuppressive drug exposure may facilitate personalized optimal drug dosing and aid in designing drug minimization strategies.
CITATION INFORMATION: Pankewycz O., Onan E., Laftavi M., Gruessner R., Smileuski H., Alissandrello E., Gruessner A. A Novel Combined Formula to Direct Optimal Immunosuppressive Drug Exposure in Renal Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Pankewycz O, Onan E, Laftavi M, Gruessner R, Smileuski H, Alissandrello E, Gruessner A. A Novel Combined Formula to Direct Optimal Immunosuppressive Drug Exposure in Renal Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/a-novel-combined-formula-to-direct-optimal-immunosuppressive-drug-exposure-in-renal-transplant-recipients/. Accessed February 21, 2026.« Back to 2018 American Transplant Congress
