A Multimodal Interrogation of Human Renal Allografts
1Weill Cornell Medicine - NYPH, New York, NY, 2CareDx, New York, NY
Meeting: 2021 American Transplant Congress
Abstract number: 394
Keywords: Gene expression, Kidney transplantation, Non-invasive diagnosis, Rejection
Topic: Clinical Science » Biomarkers, Immune Assessment and Clinical Outcomes
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Video Chat
Date: Saturday, June 5, 2021
Session Time: 7:30pm-8:30pm
 Presentation Time: 7:40pm-7:50pm
Presentation Time: 7:40pm-7:50pm
Location: Virtual
*Purpose: Development and validation of noninvasive biomarkers of kidney allograft rejection should improve transplant outcomes. CTOT-04 study validated urinary cell mRNA signature (CTOT04-Sig) for T-cell rejection (ACR). Plasma donor derived cell-free DNA (dd-cfDNA) levels were associated with antibody mediated rejection (ABMR) in a multicenter setting. We tested the hypothesis that a composite signature of CTOT04-Sig and plasma dd-cfDNA percentage is diagnostic of AR with high accuracy.
*Methods: We profiled biopsy matched 58 urine & plasma specimens from 55 graft recipients: 11 ACR, 10 AMR/mixed rejection, 22 ATI & 15 normal (Nl) biopsies. RNA from urine cell pellets was reverse transcribed to cDNA and customized PCR assays were used to measure CTOT04-Sig consisting of 18S rRNA normalized CD3E mRNA and IP-10 mRNA and 18S rRNA copies. AlloSure measurements were performed by CareDx using a targeted next-generation sequencing assay consisting of 405 single-nucleotide polymorphisms.
*Results:
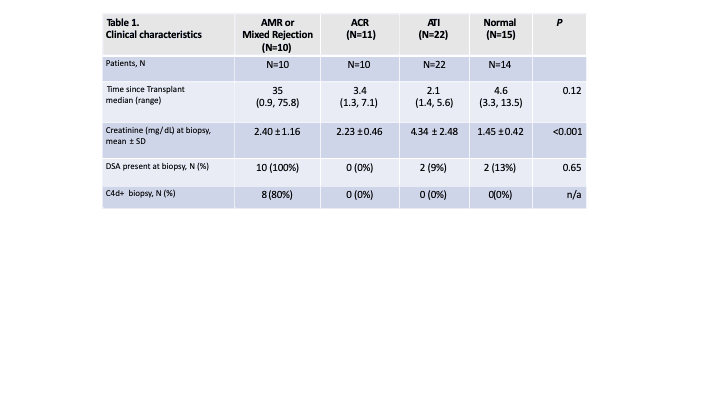
Table 1 is a summary of the study group characteristics.
Violin plots (Fig1A) show that the CTOT04-Sig score is significantly different increased in both ACR and AMR compared to ATI or Nl. dd-cfDNA is significantly increased in AMR vs. ACR, ATI or Nl (Fig1B).
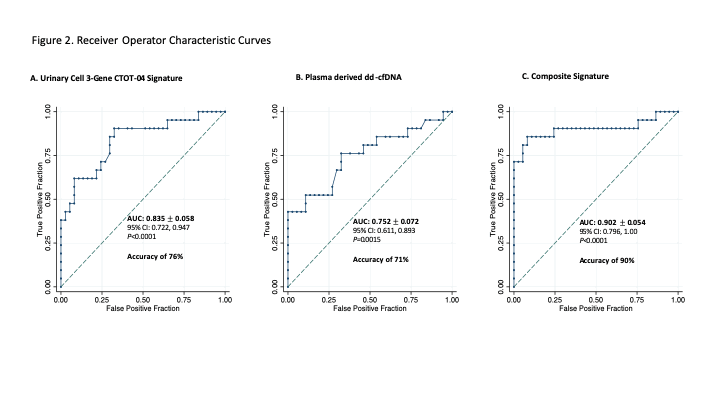
The ability of the signatures to distinguish AR (TCMR + AMR + Mixed) from No AR (ATI+ Normal) was analyzed using receiver operating characteristic (ROC) curves (Fig2). The composite signature had the highest area under the ROC (Fig2C) with a diagnostic accuracy of 90%, outperforming the CTOT04-Sig (P=0.045) and dd-cfDNA (P=0.028).
*Conclusions: Composite signature of CTOT-04 signature and dd-cfDNA allows for accurate and specific diagnosis of AR of kidney allografts and this multimodal approach should be validated in a prospective, multicenter study.
To cite this abstract in AMA style:
Dadhania D, Ding R, Xu H, Lee C, Snopkowski C, Salinas T, Muthukumar T, Lee J, Woodward R, Grskovic M, Dholakia S, Suthanthiran M. A Multimodal Interrogation of Human Renal Allografts [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/a-multimodal-interrogation-of-human-renal-allografts/. Accessed February 15, 2026.« Back to 2021 American Transplant Congress