12-Month Athena Study: Everolimus Vs. Standard Regimen in De Novo Renal Transplant Recipients
1Athena Study Group, Germany
2Novartis, Pharma, Germany.
Meeting: 2015 American Transplant Congress
Abstract number: D147
Keywords: Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 5, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Background: Post-kidney transplant (KTx) long-term and full dose/standard calcineurin inhibitor (CNI) use is associated with an increased risk for malignancies, cardiovascular disease, and renal failure. Previous studies have shown that everolimus (EVR) allowed further CNI reduction and therefore helps to preserve renal function without affecting efficacy. ATHENA study is designed to evaluate the renal function comparing EVR-based regimens with reduced CNI exposure (tacrolimus [TAC] or cyclosporine A [CsA]) vs a standard treatment protocol with mycophenolic acid (MPA) and TAC in de novo KTx (day 0) recipients (KTxR).
Methods: This is a 12-month (M), multi-center, open-label, prospective, randomized, parallel group study in KTxR (≥18 years) receiving renal allografts from deceased or living donors. Eligible patients will be randomized prior to Tx to one of the three treatment arms (1:1:1): TAC MPA+steroids (n=204) or EVR+TAC+steroids (n=204) or EVR+CsA+steroids (n=204) all with basiliximab induction. Patients with thrombocytopenia or leukopenia, uncontrolled hypercholesterolemia or hypertriglyceridemia, ABO incompatible Tx will be excluded. The primary objective is to demonstrate non-inferiority in renal function (eGFR by Nankivell formula) in one of the EVR arms compared to the TAC+MPA+steroids arm at M12 post-KTx. The key secondary objective is to assess the incidence of treatment failure (BPAR, graft loss or death) at M12 post-KTx. Other objectives are to evaluate GFR by different formulae, assess the incidence of efficacy endpoints (BPAR, graft loss and death), the incidence and severity of viral infections (CMV, BKV), the incidence and duration of delayed graft function, to evaluate left ventricular hypertrophy (by LV mass index), and to compare HLA- and non-HLA-antibody evolution.
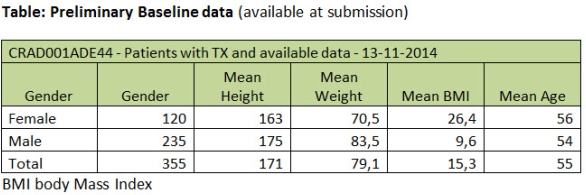
Study status: The study recruitment of this large European study is currently ongoing and 535 patients were enrolled, 404 patients in Germany and 137 in France. The preliminary results of this ongoing trial are expected in 2016.
To cite this abstract in AMA style:
Sommerer C, Suwelack B, Dragun D, Hauser I, Schenker P, Bäumer D, Nashan B, Thaiss F. 12-Month Athena Study: Everolimus Vs. Standard Regimen in De Novo Renal Transplant Recipients [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/12-month-athena-study-everolimus-vs-standard-regimen-in-de-novo-renal-transplant-recipients/. Accessed July 12, 2025.« Back to 2015 American Transplant Congress
