Polyclonal Treg Adoptive Therapy for Control of Subclinical Kidney Transplant Inflammation (TASK Pilot Trial).
UCSF, San Francisco, CA.
Meeting: 2016 American Transplant Congress
Abstract number: 412
Keywords: Inflammation, Kidney transplantation, Polyclonal, T cells
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Novel Agents
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Veterans Auditorium
Purpose: Early renal graft inflammation is associated with progressive fibrosis & late dysfunction. Treg therapy can reverse established inflammation in animal models. We conducted a pilot safety & feasibility trial of Treg therapy for subclinical kidney transplant inflammation.
Methods: Peripheral blood CD4+CD25+CD127lo/- Tregs were isolated using FACS & expanded ex vivo for 2 wks with anti-CD3/anti-CD28 stimulations/IL-2 in a deuterated glucose-containing medium to label Tregs. Stable kidney transplant recipients with subclinical inflammation on 6-mth protocol biopsies received a single infusion of 320×106 Tregs. %Deuterium enrichment by GC-MS allowed estimation of levels of infused (labeled) Tregs in circulation. Inflammation was assessed on kidney biopsies using Banff scoring and leukocyte common antigen positive (LCA+) cell density & in urine using common rejection module (uCRM), an 11-gene biomarker of acute rejection.
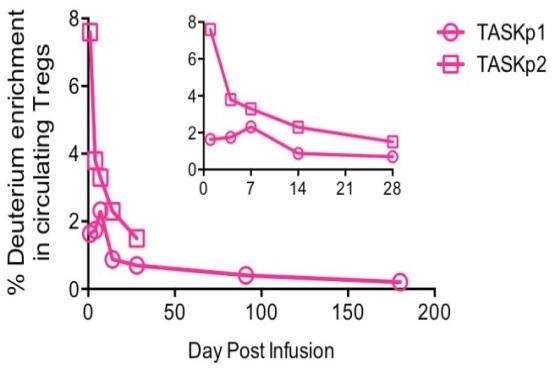
Results: Two kidney transplant recipients on tac/MMF/prednisone received Treg infusions and 6 mths of follow-up. Tregs expanded >100-fold and met all release criteria for infusion. Infused Tregs peaked within wk 1, representing up to 7.5% of all circulating Tregs (Figure). Decay of infused Tregs was similar to that noted in non-immunosuppressed patients treated with the same dose. Graft inflammation improved in both patients (Table). uCRM data are being analyzed. One patient had transient lymphopenia (day 4-21) post-infusion. No infusion reactions, infections or acute rejections were seen & graft function remained stable.
Conclusions: It is feasible to isolate & expand Tregs from transplanted patients on immunosuppression. Infused Tregs were well tolerated & had pharmacokinetics similar to those in non-immunosuppressed patients. CTOT-21 will test the efficacy of polyclonal and alloreactive Tregs for control of subclinical graft inflammation.
| Subject | Kidney Biopsy Time | Banff Score | LCA+ Cells/mm2 |
| TASKp1 | Baseline | i1 t1 | 337.2 |
| 2 weeks | i0 t0 | 18.9 | |
| 6 months | i0 t0 | 126.1 | |
| TASKp2 | Baseline | i0 t1 | 469.1 |
| 2 weeks | i0 t0 | 303.5 | |
| 6 months | i0 t1 | 74.9 |
CITATION INFORMATION: Chandran S, Tang Q, Sarwal M, Laszik Z, Putnam A, Sigdel T, Tavares E, Bluestone J, Vincenti F. Polyclonal Treg Adoptive Therapy for Control of Subclinical Kidney Transplant Inflammation (TASK Pilot Trial). Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Chandran S, Tang Q, Sarwal M, Laszik Z, Putnam A, Sigdel T, Tavares E, Bluestone J, Vincenti F. Polyclonal Treg Adoptive Therapy for Control of Subclinical Kidney Transplant Inflammation (TASK Pilot Trial). [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/polyclonal-treg-adoptive-therapy-for-control-of-subclinical-kidney-transplant-inflammation-task-pilot-trial/. Accessed February 9, 2026.« Back to 2016 American Transplant Congress
