Long-Term Kidney Allotransplant Acceptance is Defined by a Shift from a T Cell to a B Cell Rich Immune Microenvironment Within the Graft
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, 2Department of Surgery, Boston Children's Hospital, Boston, MA, 3Department of Pathology, Massachusetts General Hospital, Boston, MA, 4Harvard College, Cambridge, MA, 5Department of Surgery, Washington University, St. Louis, MO, 6Transplantation Research Center, Renal Division, Brigham and Women’s Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 601
Keywords: B cells, Kidney transplantation, Mice, Tolerance
Topic: Basic Science » Basic Science » 04 - B-cell / Antibody /Autoimmunity
Session Information
Session Time: 8:25am-9:30am
 Presentation Time: 9:15am-9:30am
Presentation Time: 9:15am-9:30am
Location: Hynes Veterans Auditorium
*Purpose: Mouse kidney allografts are spontaneously accepted in select, fully-mismatched donor-recipient strain combinations, such as DBA/2J to C57BL/6 (B6). Tolerant renal grafts develop aggregates of lymphocytes that form regulatory Tertiary Lymphoid Organs (rTLOs). Prior Nanostring analysis has provided evidence of an increasing B cell signature in accepted kidney allografts following transplantation. Depletion of Tregs at early stages (3-5 weeks post-transplant) results in acute rejection of the allograft, while depletion of Tregs at ≥ 24 weeks results in very slow or no rejection, suggesting different regulatory mechanisms are at play.
*Methods: We performed life sustaining DBA/2 to WT.B6 kidney transplants. Cells were isolated at 1 week (n=3), 3 weeks (n=5), and 24 weeks (n=3) post-transplant. Intrarenal CD45+ cells were sorted and analyzed by scRNAseq. The cDNA libraries were processed via Next Generation Sequencing and the data was analyzed using the Seurat software package in Rstudio. Additionally, flow cytometric and IHC analyses were performed to further characterize the intragraft B cell population.
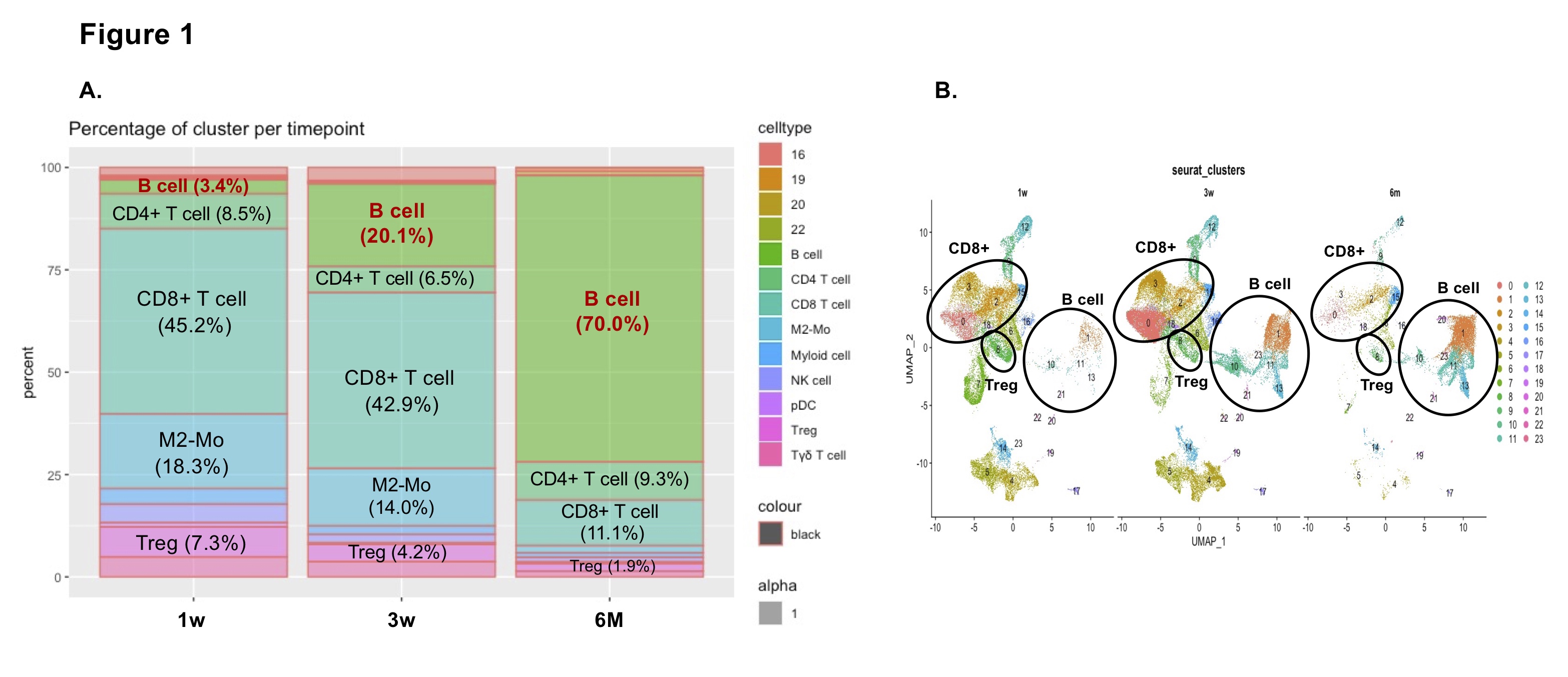
*Results: scRNAseq analysis shows a shift from a T cell to a B cell rich immune environment within the graft by 24 weeks post-transplantation (Fig. 1A, 1B). Flow cytometric analysis of intrarenal B cells reveals that Tim-1+CD1d+CD5+ are present at 20 weeks post-transplantation. Gene expression analysis shows that regulatory B cell markers appear as early as 3 weeks post-transplantation and are amplified by 24 weeks. IHC staining shows the emergence of a germinal center-like structures within the graft at 8 weeks, with stable expression of genes that dictate a GC program by 24 weeks.
*Conclusions: We show a shift from a T cell to a B cell rich environment in long-term accepted kidney allografts which suggests a transition from a Treg to a Breg mode of immune regulation.
To cite this abstract in AMA style:
Szuter ES, Yokose T, Guinn MT, Ge J, Rosales I, Kwon B, Russell PS, Cuenca A, Kreisel D, Sage P, Madsen JC, Colvin RB, Alessandrini A. Long-Term Kidney Allotransplant Acceptance is Defined by a Shift from a T Cell to a B Cell Rich Immune Microenvironment Within the Graft [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-kidney-allotransplant-acceptance-is-defined-by-a-shift-from-a-t-cell-to-a-b-cell-rich-immune-microenvironment-within-the-graft/. Accessed February 14, 2026.« Back to 2022 American Transplant Congress