Technology-Driven Performance Improvement of a Biomarker Panel in Kidney Transplant: OmniGraf
Eurofins, Framingham, MA
Meeting: 2022 American Transplant Congress
Abstract number: 1569
Keywords: Gene expression, Polymerase chain reaction (PCR), Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Analyze the impact of the transition from TruGraf microarray to TruGraf PCR on the complementary performance characteristics of TruGraf plus TRAC (OmniGraf).
*Methods: Development of the TruGraf assay was originally performed via a high-throughput analysis of gene expression on a microarray system. Microarray, while useful as a discovery tool, is a source of high assay variability and can have results influenced by the complex hybridization assay. It is also time-consuming, labor-intensive, and requires large amounts of pure RNA. This leads to a turnaround time of at least 7 days. Using RT-PCR and microfluidics (Fluidigm Biomark HD) provides more rapid and quantitative analysis of gene expression, while requiring less RNA input and substantially reducing turnaround time. We performed analytical and clinical validation between the RT-PCR and microarray processes. TruGraf results of “TX” are considered negative and “not-TX” are considered positive while TRAC results of <0.7% are considered negative and results of ≥ 0.7% are considered positive.
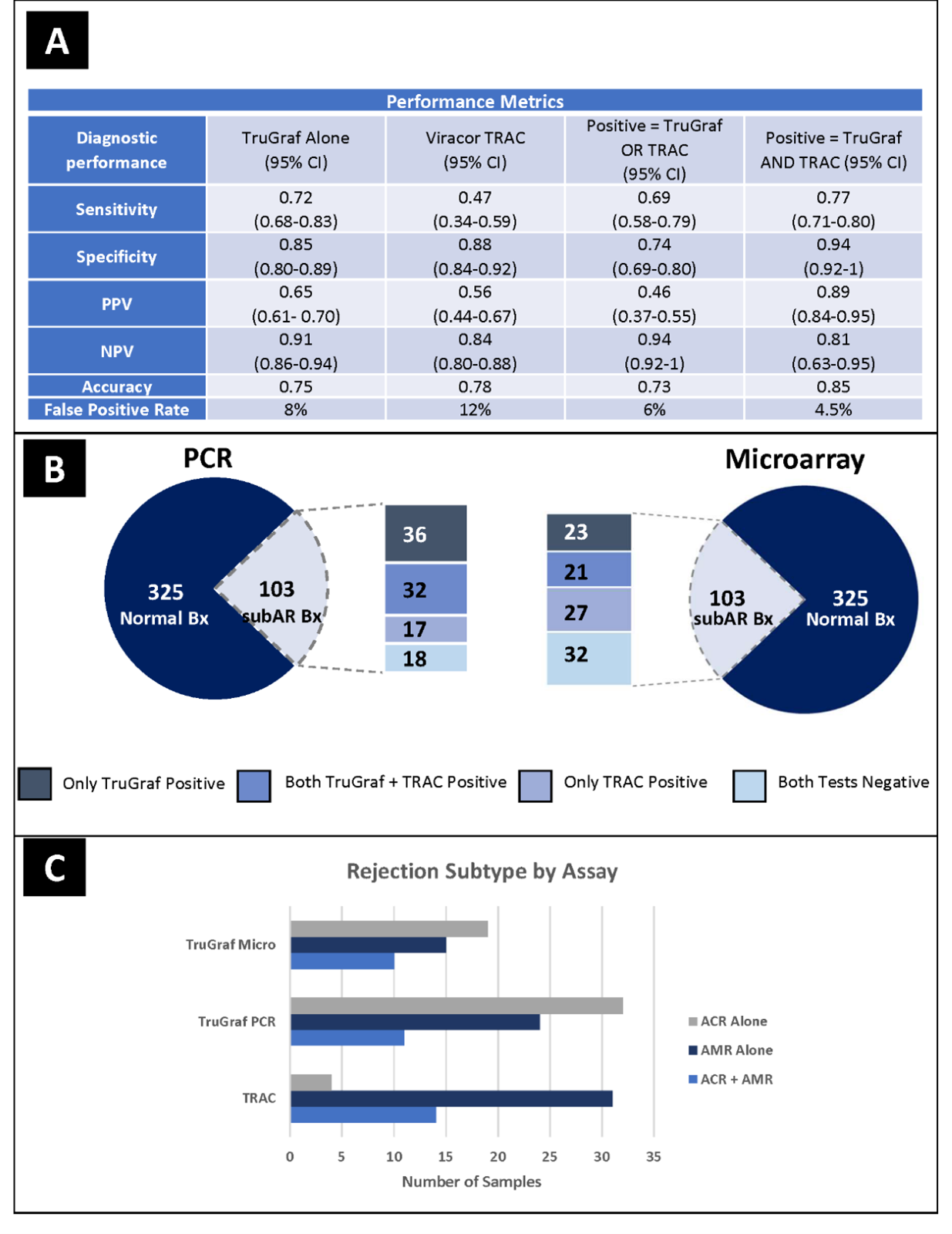
*Results: The NPV increased from 88% to 94% when both assays were negative. The PPV increased from 81% to 89% when both tests were positive (Figure 1A/B). False negative results were reduced from 31% to 17%, while true negative results improved from 74% to 81%. 26.2% of results were positive for one test and negative for the other: 11.7% showed elevated TRAC (+) and a TX TruGraf (-); 14.5% showed a TruGraf not-TX (+) and low TRAC score (-). A previous report noted that TruGraf was significantly better at detecting subclinical TCMR and TRAC was significantly better at detecting subclinical ABMR; however, this improvement in TruGraf technology increased its detection of all subtypes of rejection (Figure 1C).
*Conclusions: In the field of transplant biomarkers where high NPV values have been the focus we present novel clinical validation data on the first commercial biomarker panel with a high PPV. With the data presented here, TruGraf PCR plus TRAC (OmniGraf) results provide a high probability of either immune quiescence or subclinical rejection to support clinical decision-making.
To cite this abstract in AMA style:
West-Thielke P, Miller C, Grund N, Sutton A, Sinha R, Miller M, Kleiboeker S, Weems J. Technology-Driven Performance Improvement of a Biomarker Panel in Kidney Transplant: OmniGraf [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/technology-driven-performance-improvement-of-a-biomarker-panel-in-kidney-transplant-omnigraf/. Accessed February 9, 2026.« Back to 2022 American Transplant Congress