The Promise Of Tolerance In Living Donor Kidney Transplant (LDKT): A Retrospective, Real-world Assessment Of The Safety And Efficacy Of LDKT With FCR001 Investigational Cell-therapy Compared With Standard Of Care (SOC)
1Talaris Therapeutics, Inc, Wellesley, MA, 2SURGERY, Northwestern Medicine, Chicago, IL, 3Comprehensive Transplant Ctr, Chicago, IL, 4Evidera, Bethesda, MD, 5Talaris Therapeutics, Inc., Louisville, KY, 6University of Iowa, Iowa City, IA
Meeting: 2022 American Transplant Congress
Abstract number: 9090
Keywords: Immunosuppression, T cells, Tolerance
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: FCR001 (FCR) is a cell therapy derived from mobilized donor PBMCs including hematopoietic progenitor cells, facilitating cells, and αβ T cells, intended to establish durable chimerism and donor-specific tolerance without immunosuppression (IS). A retrospective cohort study evaluated the efficacy and safety of LDKT with FCR/ IS-withdrawal with contemporaneous standard of care (SOC) (IS: Tac + MPA).
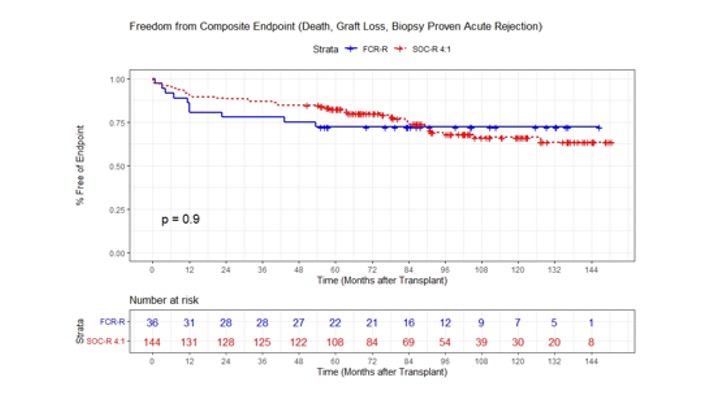
*Methods: FCR-treated LDKTs (n=36) with IS withdrawal planned at Month 12 were compared to 4:1 propensity-score matched were matched on age, HLA mismatch, PRA, BMI, and other characteristics OC-LDKTs (n=144 in the Northwestern Enterprise Data Warehouse (EDW) with 60 month follow-up. FCR-eligible SOC patients (age ≥18, HLA 0-6 mismatch, PRA ≤20%, BMI 18-36 kg/m2). The incidence of the composite primary endpoint (biopsy-proven acute rejection (BPAR), graft loss, or death at 24 months post-LDKT) and secondary endpoints (eGFR and diabetes (DM) after transplant defined using ICD-9/10 codes) were assessed.
*Results: Among FCR patients, 72% were off all IS at 24 months. At 24 months, 88.9% of SOC and 77.8% of FCR (p=NS) were free from BPAR, death, or graft loss There was no difference in freedom from BPAR at 24 months (92.3% vs. 80.3%, p=0.09) and at 60 months (88.6%vs. 80.3%, p=NS). No FCR patient had BPAR after IS withdrawal.
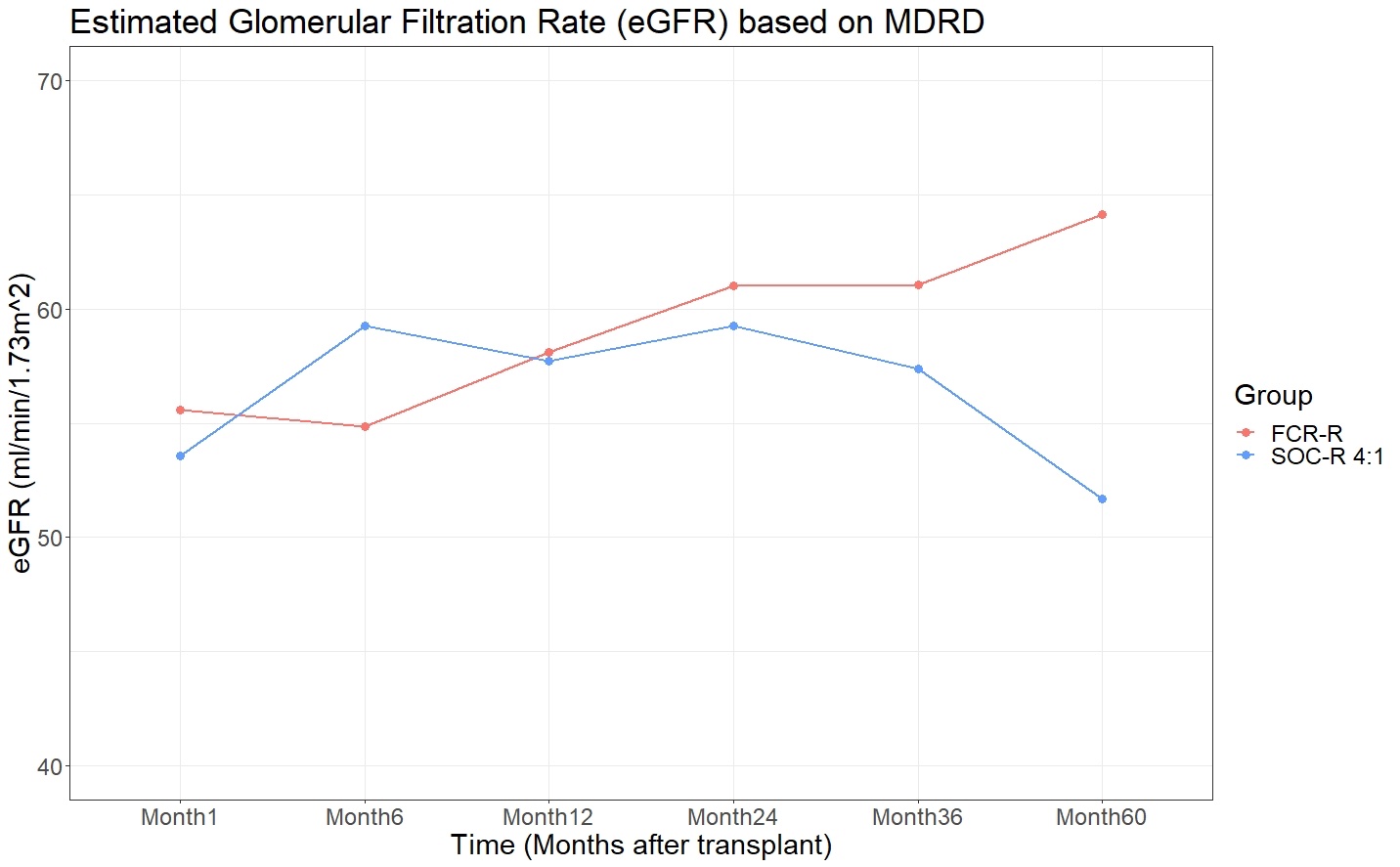
Mean eGFR was similar in FCR vs. SOC patients at 12 months (58.1 vs. 57.7 mL/min, p=NS and 24 months (61.0 vs. 59.3 mL/min, p=NS), and higher at 60 months (64.1 vs. 51.7 mL/min, p=0.02).
Overall, fewer FCR patients experienced DM (FCR: 5.6% vs. SOC: 18.1%, P=NS).
*Conclusions: LDKT with FCR treatment permitted withdrawal of all IS in 72% of recipients. LDKT with FCR did not increase the risk of BPAR, graft loss, or death compared with SOC, but was associated with preserved long term allograft function.
To cite this abstract in AMA style:
Krieger N, Leventhal JR, Ho B, Richards M, Schaumberg D, Ildstad S, Axelrod D. The Promise Of Tolerance In Living Donor Kidney Transplant (LDKT): A Retrospective, Real-world Assessment Of The Safety And Efficacy Of LDKT With FCR001 Investigational Cell-therapy Compared With Standard Of Care (SOC) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/the-promise-of-tolerance-in-living-donor-kidney-transplant-ldkt-a-retrospective-real-world-assessment-of-the-safety-and-efficacy-of-ldkt-with-fcr001-investigational-cell-therapy-compared-with-stan/. Accessed February 15, 2026.« Back to 2022 American Transplant Congress