Utility of Donor Derived Cell-Free DNA in Liver Transplantation
Surgery, University of Florida, Gainesville, FL
Meeting: 2022 American Transplant Congress
Abstract number: 1557
Keywords: Liver transplantation, Monitoring, Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: As a non-invasive measure of allograft injury, quantifying the fraction of donor-derived cell-free DNA (dd-cfDNA) has proven to be useful technique in kidney transplantation. Its utility in liver transplantation, however, remains unproven. Specifically, whether the dd-cfDNA fraction adds any additional information on allograft status over the standard liver function tests (LFTs) is unclear.
*Methods: We evaluated the correlation of dd-cfDNA with standard postoperative LFTs after liver transplantation in a prospectively collected cohort of patients at the University of Florida. Adult recipients of orthotopic whole liver grafts were enrolled under an IRB approved protocol. Dd-cfDNA was measured in up to fifteen plasma samples obtained at the same time as standard-of-care LFT measurements between two weeks and one year after transplantation. The study included patients with stable liver function, as well as patients being evaluated for rejection or infection. 38 patients were enrolled and 112 specimens were tested for dd-cfDNA fraction.
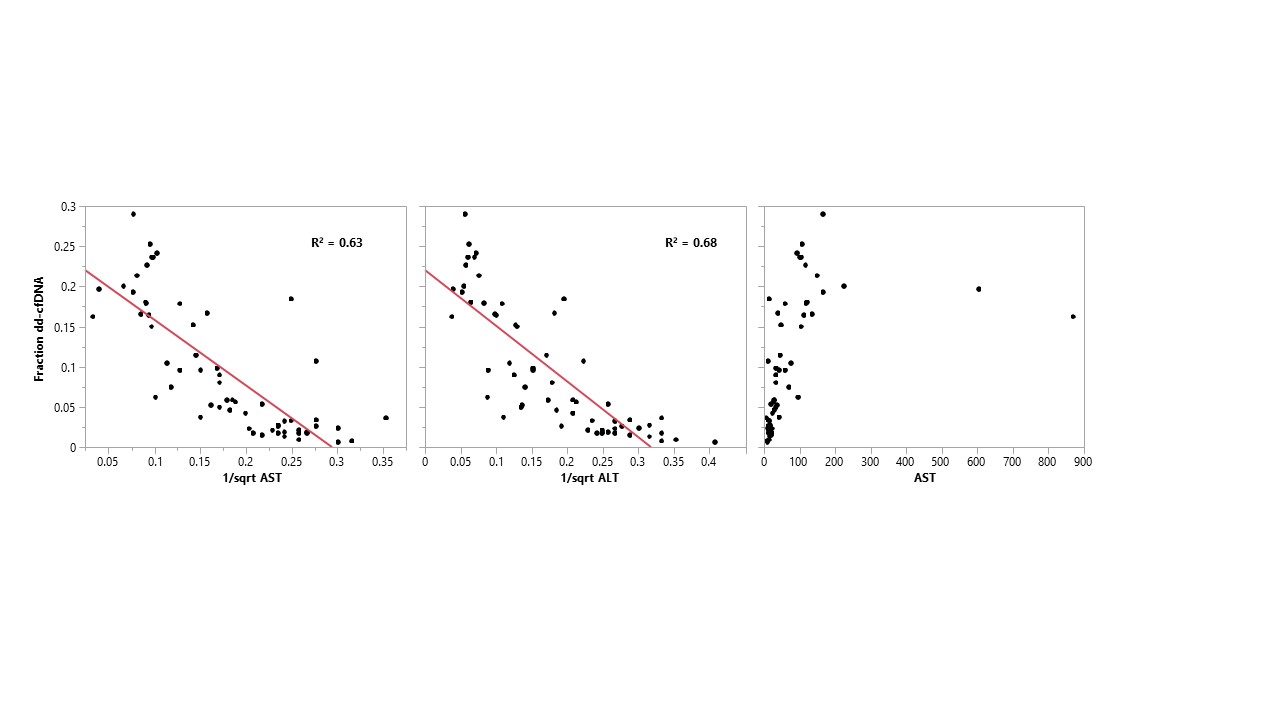
*Results: In 28 samples with LFTs all within normal limits, dd-cfDNA fractions were below 10%. Conversely, all samples with dd-cfDNA fraction greater than 10% had either elevated alanine aminotransferase (ALT) or alkaline phosphatase. There was a close correlation between dd-cfDNA % and 1/sqrt(AST) or 1/sqrt(ALT) (R2 = 0.63 and 0.68, respectively). When examined on an individual patient basis, this correlation was tighter with few exceptions.
*Conclusions: Given these findings, dd-cfDNA quantification does not appear to provide additional sensitivity over standard LFTs for routine monitoring of post-transplant allograft injury in liver transplantation.
To cite this abstract in AMA style:
Zarrinpar A, Lewis D, Warren C, Duarte S. Utility of Donor Derived Cell-Free DNA in Liver Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/utility-of-donor-derived-cell-free-dna-in-liver-transplantation/. Accessed February 19, 2026.« Back to 2022 American Transplant Congress