Monitoring of Kidney Allograft Rejection Using Patient-Centric State-of-the-Art Tools – An Approach to Early Diagnosis and Intervention
1Internal Medicine, Nephrology, University of Texas Medical Branch, Galveston, TX, 2University of Texas Medical Branch, Galveston, TX, 3Nephrology, University of Texas Medical Branch, Houston, TX, 4Surgery, University of Texas Medical Branch, Galveston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1680
Keywords: Gene expression, HLA antibodies, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » 34 - Kidney: Acute Cellular Rejection
Session Information
Session Name: Kidney: Acute Cellular Rejection
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The early detection and timely management of kidney allograft rejection using traditional markers including serum creatinine (SCr) has been inadequate due to lagging response. Donor-derived cell-free DNA (dd-cfDNA) is a noninvasive diagnostic tool that may safely and quantitatively assess allograft rejection. In addition, the Molecular Microscope (MMDx) tissue gene expression may offer increased precision to traditional histology variations on kidney biopsy. We here at University of Texas Medical Branch utilize these state-of-the-art diagnostic tools and monitor at specified time intervals for early detection and management of kidney allograft rejection.
*Methods: This single-center retrospective study included kidney transplant recipients who were monitored for rejection at specified time intervals by utilizing Scr, HLA donor-specific antibodies (DSA), and dd-cfDNA. The clinically indicated allograft biopsy was performed in accord and sample was sent for both histopathology and MMDx. The SCr, dd-cfDNA, and DSAs were assessed pre-intervention at the time of biopsy and post-intervention at last follow up. Patients were categorized into rejection (Rej) group if they had evidence of rejection on traditional histology or MMDx (either antibody-mediated [ABMR], T-cell mediated [TCMR], or mixed) and non-rejection (NRej) group.
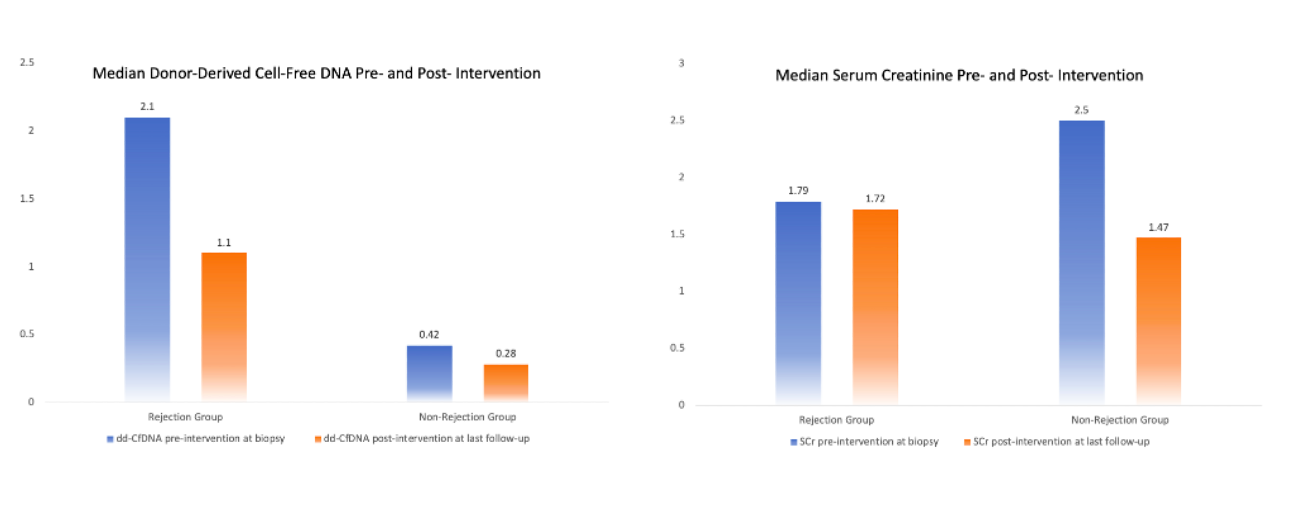
*Results: A total of 101 patients (53±13 years) were included. The Rej group included 61 patients (51±13 years) and NRej group included 40 patients (56±12 years). The DSAs were positive in 49% patients in the Rej group and 20% patients in the NRej group. Among the Rej group, pre-intervention median (IQR) dd-cfDNA was found to be 2.1% (0.67%, 3.75%) and post-intervention was 1.1% (0.39%, 2.10%). Among NRej group, the pre-intervention dd-cfDNA was found to be 0.42% (0.23%, 0.72%) and post-intervention was 0.28% (0.12%, 0.40%) (Figure). Among the Rej group, traditional histology was found to be negative for rejection in 9 patients (15%) and MMDx was negative in 8 patients (13%).
*Conclusions: The utilization and monitoring of noninvasive state-of-the-art tools including dd-cfDNA and DSAs along with addition of MMDx to kidney biopsy can add precision to traditional histology for early detection and timely management of kidney allograft rejection.
To cite this abstract in AMA style:
Rizvi A, Gamilla-Crudo A, Hussain SA, Kueht M, Fair J, Mujtaba M. Monitoring of Kidney Allograft Rejection Using Patient-Centric State-of-the-Art Tools – An Approach to Early Diagnosis and Intervention [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/monitoring-of-kidney-allograft-rejection-using-patient-centric-state-of-the-art-tools-an-approach-to-early-diagnosis-and-intervention/. Accessed February 21, 2026.« Back to 2022 American Transplant Congress