Safety and Immunogenicity of BNT-162b2 versus Chadox1 Vaccine in Solid Organ Transplant Recipients: Prospective Study
1Pharmaceutical Care Division - Organ Transplant Centre of Excellence, King Faisal Specialist Hospital and Research Centre (KFSHRC), Riaydh, Saudi Arabia, 2Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 3Medical Laboratory, KFSHRC, Riaydh, Saudi Arabia, 4Medical Microbiology, KFSHRC, Riaydh, Saudi Arabia, 5Transplant Coordination Team - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 6Transplant Coordination Team - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 7Kidney & Pancreas Health Centre, KFSHRC, Riaydh, Saudi Arabia, 8Analytics Data Centre - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 9Data Management - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 10Infectious Diseases - Medicine Department, KFSHRC, Riaydh, Saudi Arabia, 11Abdominal Transplant & Hepatobiliary Surgery Centre, KFSHRC, Riaydh, Saudi Arabia, 12Data Management. - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 13Liver & Small Bowel Health Centre, KFSHRC, Riaydh, Saudi Arabia, 14Infectious Diseases - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia, 15Biostatistics, Epidemiology &Scientific Computing, KFSHRC, Riaydh, Saudi Arabia, 16Histocompatibility and Immunogenetics Laboratory Kidney & Pa - Organ Transplant Centre of Excellence, KFSHRC, Riaydh, Saudi Arabia
Meeting: 2022 American Transplant Congress
Abstract number: 1657
Keywords: COVID-19, Kidney transplantation, Liver transplantation, Vaccination
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
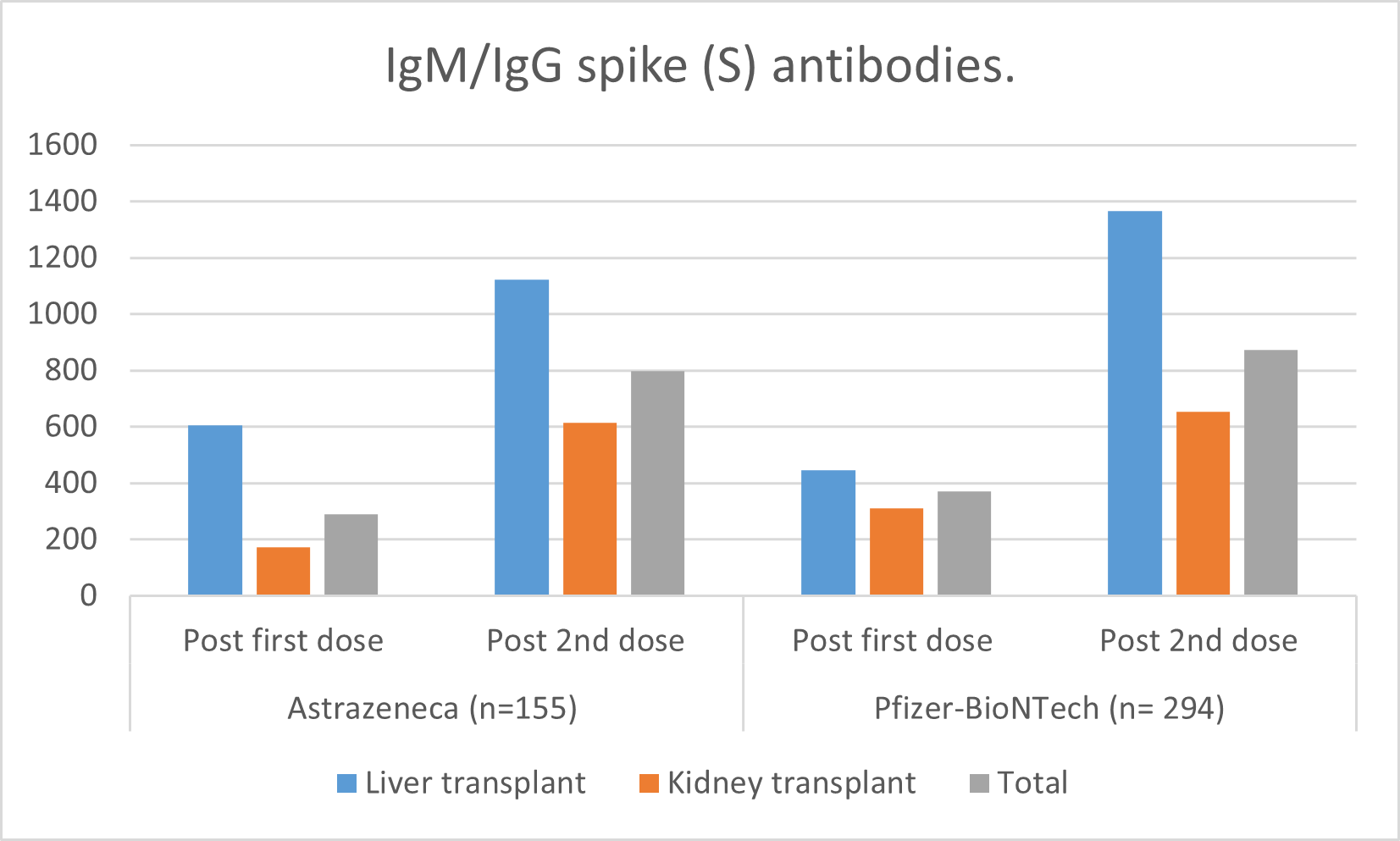
*Purpose: Data showed seroconversion after different SARS-CoV-2 vaccination platforms might yield diminished response in transplant recipients. However, it is unknown whether different vaccination platforms could offer a specific grade of protection against SARS-CoV-2.
*Methods: we prospectively studied adult kidney & liver recipients who received who had no previous COVID-19 infection, and received either ChAdOx1 or BNT-162b2 vaccines between January 2021 to September 2021, with an assessment of IgM/IgG spike (S) antibodies.
*Results: Our cohort is composed of kidney (n=235) or liver (n=217) patients, who have received either ChAdOx1 (N=157) or BNT-162b2 (n=295). The response was higher with mRNA vaccine. Unresponsiveness is found to be mainly linked to diabetes and older age. Side effects were similar to those reported in clinical trials.
*Conclusions: mRNA vaccines might elicit a higher humoral immunity response as compared with ChAdOx1 in immunosuppressed transplant patients.
To cite this abstract in AMA style:
Ajlan A, Ali T, Aleid H, Almeshari K, Alkaff M, Althuwaidi S, Fajji L, Alali A, Halabi D, Ullah A, Marquez KH, Elmikkaoui B, Alrajhi A, Zidan A, Albogumi E, Bzeizi K, Almaghrabi R, DeVol E, Al-Awwami M, Alghamdi S, Broering P. Safety and Immunogenicity of BNT-162b2 versus Chadox1 Vaccine in Solid Organ Transplant Recipients: Prospective Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-immunogenicity-of-bnt-162b2-versus-chadox1-vaccine-in-solid-organ-transplant-recipients-prospective-study/. Accessed January 13, 2026.« Back to 2022 American Transplant Congress