Multicenter Effort Assessing the Effects of COVID Infection and Vaccination on HLA Antibodies in Waitlisted Patients with ESRD
1UCSF Medical Center, San Francisco, CA, 2MedStar Georgetown Transplant Institute, Washington, DC, 3Emory University Hospital, Atlanta, GA, 4Pathology and Laboratory Medicine, University of Texas Health San Antonio, San Antonio, TX, 5Houston Methodist, Houston, TX, 6University of Alabama, Birmingham, AL, 7Hospital Univ of Pennsylvania, Philadelphia, PA, 8University of Alabama at Birmingham, Birmingham, AL, 9Surgery, Keck Medicine of USC, Los Angeles, CA, 10University of Alabama at Birmingham Hospital, Birmingham, AL, 11University of Pennsylvania, Philadelphia, Pe, 12Emory University School of Medicine, Atlanta, GA, 13UT Health San Antonio, San Antonio, TX, 14Univ of California-San Francisco, San Francisco, CA, 15Houston Methodist Hospital, Houston, TX, 16Immunogenetics and Transplantation Laboratory (ITL), San Francisco, CA
Meeting: 2022 American Transplant Congress
Abstract number: 405
Keywords: COVID-19, HLA antibodies, Vaccination, Waiting lists
Topic: Clinical Science » Kidney » 44 - Kidney Acute Antibody Mediated Rejection
Session Information
Session Name: Kidney Immunosuppression: Desensitization & Acute Antibody Mediated Rejection
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:20pm-4:30pm
Presentation Time: 4:20pm-4:30pm
Location: Hynes Ballroom A
*Purpose: Although rare, infection and vaccination have been reported to result in human leukocyte antibodies (HLA). We analyzed the effect of SARS-CoV2 infection, or COVID-19 vaccination on HLA antibodies.
*Methods: Multicenter study of waitlisted renal transplant patients (pts) either infected with SARS-CoV2, or fully vaccinated against it with single antigen testing within 3 months prior, and 3 months after. Demographics and infection/vaccination details were collected. If the calculated panel reactive antibody (cPRA) changed specificities were collected. Pts with cPRA change were adjudicated by each center’s HLA laboratory director and coordinating center’s lab director.
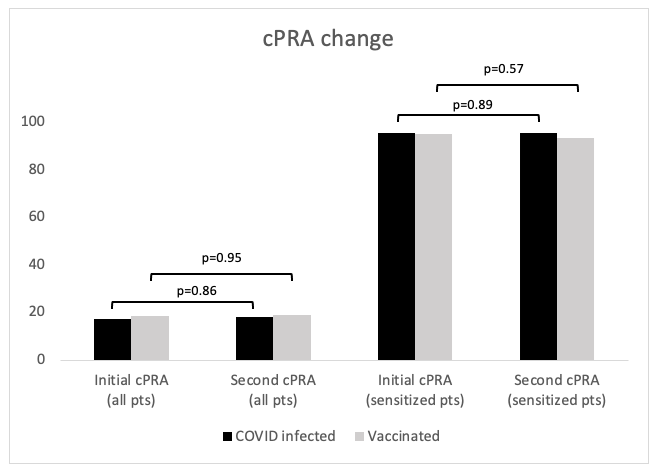
*Results: Data from 5 centers, 409 pts with single antigen testing before and after infection or vaccination was analyzed. 282 pts had an initial cPRA of 0%, and 72 had an initial cPRA>80%. 30 pts (7.0%) had a change in cPRA, 17 (3.9%) had an increase and 13 (3.0%) a decrease. After adjudication no pts with a clinically significant HLA antibody specificity increase were identified. The differences have been largely influenced by one or two specificities with subtle fluctuations around the borderline of the participating centers’ mean fluorescence intensity cutoff for unacceptable antigen listing.
*Conclusions: To date, no clinically significant change in cPRA was seen in pts who were infected with SARS-CoV2 and recovered, nor pts fully vaccinated against COVID-19. This has implications for crossmatching at the time of organ offer after SARS-CoV2 infection or vaccination. Also, vaccination appears to be safe for waitlisted pts, even if they are sensitized.
To cite this abstract in AMA style:
Roll GR, Cooper M, Gebel H, Hitchman KM, Hobeika M, Houp J, Kamoun M, Killian J, Kim J, Kumar V, Levine M, Lunow-Luke T, Parsons R, Ranch D, Stock P, Yi S, Rajalingam R. Multicenter Effort Assessing the Effects of COVID Infection and Vaccination on HLA Antibodies in Waitlisted Patients with ESRD [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/multicenter-effort-assessing-the-effects-of-covid-infection-and-vaccination-on-hla-antibodies-in-waitlisted-patients-with-esrd/. Accessed February 20, 2026.« Back to 2022 American Transplant Congress