Posoleucel as Preemptive Therapy for BKV Infection in Kidney Transplant Recipients: Safety and Tolerability in a Phase 2 Trial
1Inova Transplant Center, Falls Church, VA, 2U. of Pittsburg Medical Center, Harrisburg, PA, 3UT Southwestern Medical Center, Dallas, TX, 4Northwestern U. Feinberg School of Medicine, Chicago, IL, 5NYU Langone Transplant Institute, New York, NY, 6Northwell Health, Manhasset, NY, 7U. of Pittsburgh Medical Center, Pittsburgh, PA, 8Duke U. School of Medicine, Durham, NC, 9AlloVir, Cambridge, MA, 10Brigham & Women's Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 387
Keywords: Infection, Kidney transplantation, Polyma virus, T cells
Topic: Clinical Science » Infection Disease » 26 - Kidney: Polyoma
Session Information
Session Time: 8:25am-9:30am
 Presentation Time: 8:30am-8:45am
Presentation Time: 8:30am-8:45am
Location: Hynes Veterans Auditorium
*Purpose: Kidney transplant (KT) recipients with BK viremia are at risk for BK virus (BKV) nephropathy and graft loss. There are no approved therapies for BKV infection. Posoleucel (PSL) is an off-the-shelf, allogeneic multivirus-specific T cell therapy. In a phase 2 trial of PSL in hematopoietic cell transplant recipients, 100% (27/27) of those with BKV disease had a clinical response.
*Methods: We are conducting a phase 2 double-blind study of PSL in KT recipients with BK viremia (NCT04605484) in which patients are randomized 1:1:1 to receive PSL (Groups 1 and 2) or placebo (PBO) for 12 weeks. PSL is infused once a week for 3 weeks, then every 14 days (Group 1) or every 28 days (Group 2). The primary objective is safety and tolerability with a key secondary objective to assess changes in BK viremia in recipients of PSL vs PBO.
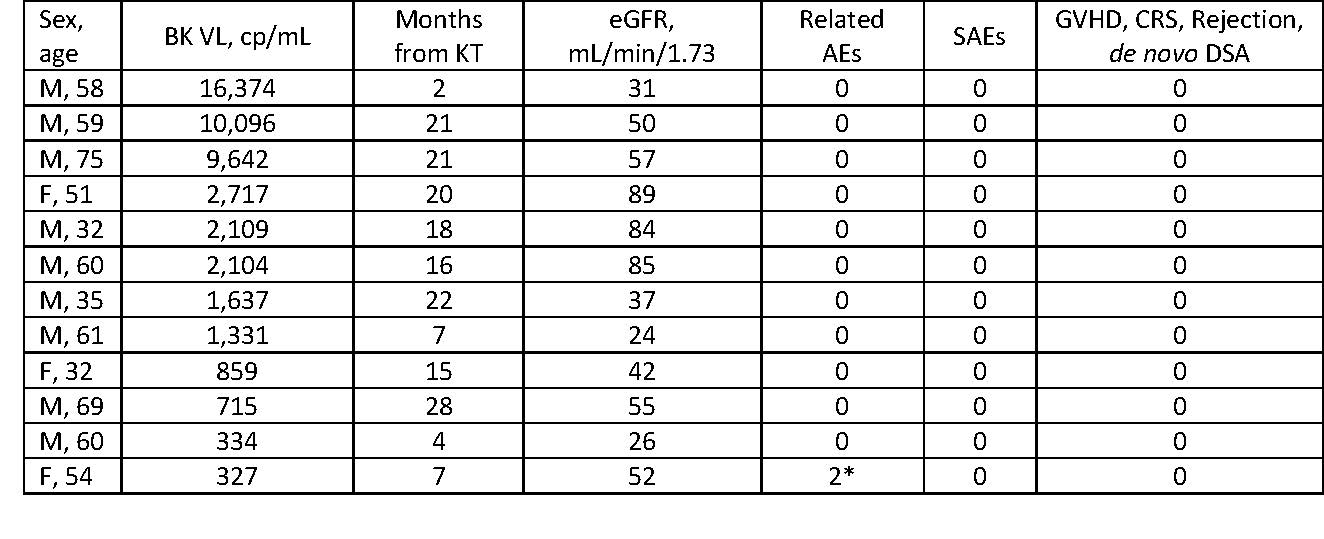
*Results: The table shows baseline characteristics and safety events to date in the first 12 patients: 75% were male, median age 58.5 years, median follow-up 85 (range 41-169) days. In these blinded data, there have been very few and only transient grade 1 treatment-related adverse events (AEs), and no graft-versus-host disease, cytokine release syndrome, graft rejection, discontinuations due to AEs, or de novo donor-specific antibodies. Kidney allograft function was stable with mean change in eGFR of -0.4 mL/min/1.73m2. Analyses of PSL’s effect on other immune and viral endpoints will be presented.
*Conclusions: In this ongoing trial—the first randomized, double-blind placebo-controlled therapeutic trial of virus-specific T cell therapy in KT recipients with BK viremia—PSL was safe and well tolerated, supporting its continued evaluation as a preemptive therapy in KT recipients at risk for BKV nephropathy.
To cite this abstract in AMA style:
Wali RK, Singh M, Wojciechowski D, Ansari MJ, Lonze B, Nair V, Sharma A, Knechtle S, Cardarelli F, Chandraker A. Posoleucel as Preemptive Therapy for BKV Infection in Kidney Transplant Recipients: Safety and Tolerability in a Phase 2 Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/posoleucel-as-preemptive-therapy-for-bkv-infection-in-kidney-transplant-recipients-safety-and-tolerability-in-a-phase-2-trial/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress