Variability In Plasma Donor-derived Cell-free DNA Levels With CLAD More Than 5-years After Lung Transplantation: Pilot Data
1Department of Medicine, University of Texas, San Antonio, San Antonio, TX, 2Natera, Inc., Ausin, TX
Meeting: 2022 American Transplant Congress
Abstract number: 9078
Keywords: Graft survival, Lung transplantation
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Donor-derived cell-free DNA (dd-cfDNA) has been clinically validated for detection of acute rejection (AR) in lung transplant (LT) although studies have typically focused on the period soon after LT. Yang et al. (2021) reported median nadir dd-cfDNA in stable patients of 0.21% at 4-mos post-LT, with an increase to 0.60-0.68% at 18-24 months. Only limited data has been reported regarding dd-cfDNA plasma levels in long term survivors.
*Methods: A single-center, cross-sectional cohort study of LT recipients (LTR) with chronic lung allograft dysfunction (CLAD) ≥5-years post-LT was performed. Plasma dd-cfDNA fractions (dd-cfDNA%; the Prospera™test; Natera, Inc.) and quantity (dd-cfDNA copies/mL) were assessed. LTR clinical status was categorized as either Complicated (C-CLAD) or Uncomplicated (U-CLAD). C-CLAD was defined as clinical presence of concurrent allograft infection, antibody-mediated rejection (AMR), or chronic severe GER / aspiration. Data is presented as median (25-75% IQR) and cohorts analyzed by Mann-Whitney-U test (p<0.05).
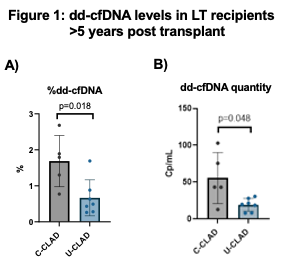
*Results: The 12 LTR (bilateral=11, unilateral=1) in this cohort were comprised of: CF (2), IPF (4), COPD / bronchiectasis (4), Re-LT (1), IPAH (1). At the time of dd-cfDNA assessment the median time after LT for native lung disease was 2,149 days (1899-2920). The C-CLAD cohort was determined on the basis of chronic aspiration (1), AMR (3), infection (1). Median dd-cfDNA (%) for the C-CLAD cohort (N=5) was 1.790% (IQR: 1.035-2.285), which was significantly elevated compared to the U-CLAD cohort (N=7; 0.490%; 0.280-0.880) (p=0.0178) (Figure 1A). dd-cfDNA quantity was also elevated in the C-CLAD cohort (43.20 cp/mL; 27.91-89.28) versus U-CLAD cohort (19.63 cp/mL; 8.15-27.90) (p=0.048) (Figure 1B).
*Conclusions: Cross-sectional analysis of patients with CLAD more than 5-years after LT shows significant variability in both dd-cfDNA fraction and quantity (cp/mL) based on the presence or absence of allograft co-morbidities (C-CLAD). By contrast, the dd-cfDNA fraction in U-CLAD appears similar to previously reported values for stable and healthy LTR.
We speculate that the presence of elevated dd-cfDNA in CLAD may heighten a clinical suspicion for co-morbidities that may respond to additional forms of therapy. Additionally, quantification of dd-cfDNA (cp/mL) may offer additional insights and precision for longitudinal assessment accompanying treatment algorithms.
To cite this abstract in AMA style:
Levine D, Ross D. Variability In Plasma Donor-derived Cell-free DNA Levels With CLAD More Than 5-years After Lung Transplantation: Pilot Data [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/variability-in-plasma-donor-derived-cell-free-dna-levels-with-clad-more-than-5-years-after-lung-transplantation-pilot-data/. Accessed January 9, 2026.« Back to 2022 American Transplant Congress