Standard Induction with Basiliximab versus no Induction in Low Immunological Risk Kidney Transplant Recipients: Study Protocol for a Randomized Controlled Trial
1KFSHRC, RIYADH, Saudi Arabia, 2MOH, RIYADH, Saudi Arabia
Meeting: 2022 American Transplant Congress
Abstract number: 1378
Keywords: Immunosuppression, Induction therapy, Interleukin-2 receptor, Rejection
Topic: Clinical Science » Kidney » 37 - Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: To compare different induction therapeutic strategies with 2 doses of basiliximab vs. no induction in low immunologic risk kidney transplant recipients as per KFSHRC protocol.
*Methods: Prospective, randomized, double blind, non-inferiority, controlled clinical trial. Hypothesis No induction is non-inferior to standard dose induction with basiliximab in low immunological risk kidney transplant recipients. The estimated total sample size will be 140 patient. A margin of non-inferiority is proposed such that the true rejection rate among those treated without Simulect® would not be more than 15%. Primary outcomes: Biopsy-proven acute rejection within first year following transplant. Secondary outcomes: a. Patient and graft survival at 1 year b. Emergence of de novo donor-specific antibodies (DSAs).
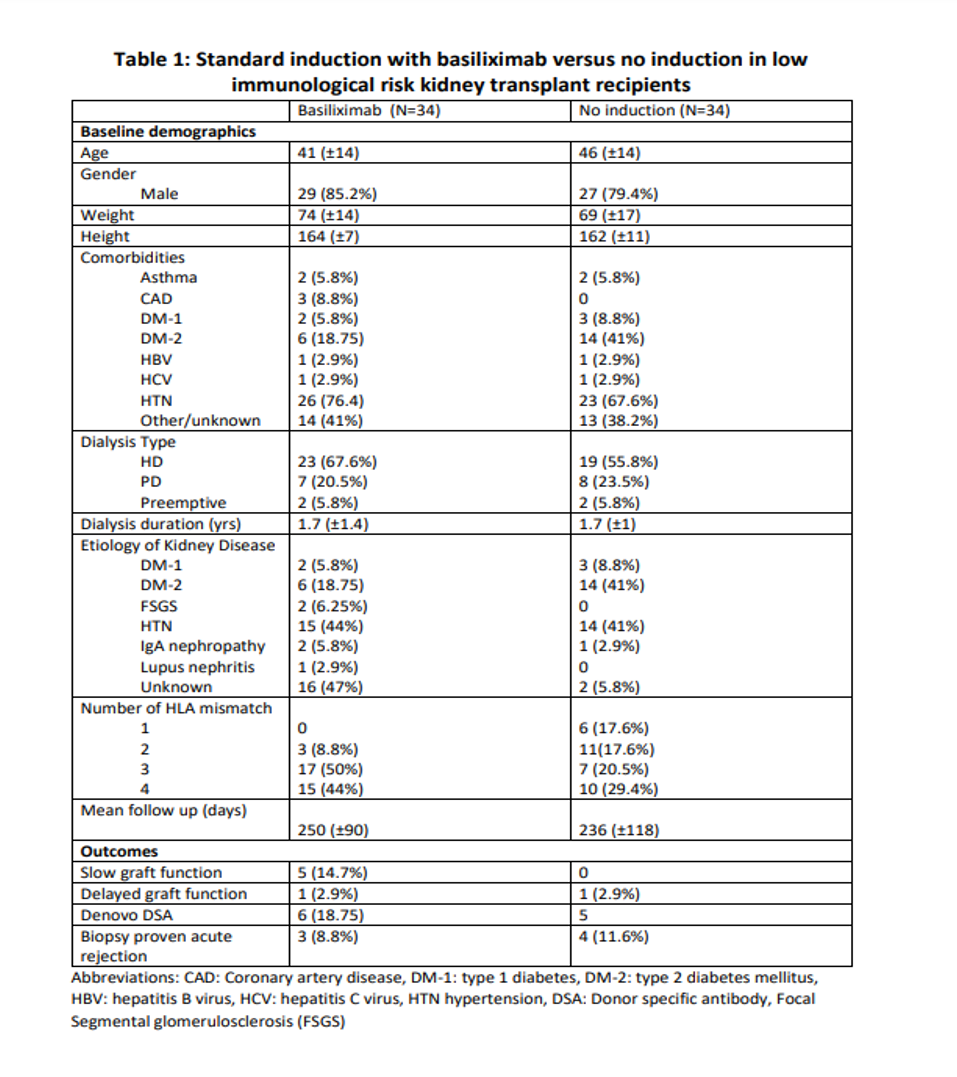
*Results: A total of 68 patients have been recruited (34 in each arm). Baseline demographics are described in table 1. biopsy proven rejection occurred in 3 patients in the basiliximab arm (8.8%) vs. 4 patient in the no induction arm (11.6%). Delayed graft function occurred in 1 patient in each arm (2.9%). Denovo DSA detected in 6 (18.75%) patients in the basiliximab arm, and 5 in the no induction arm (14.7%)
*Conclusions: Our preliminary analysis suggests that, in low immunological risk subject induction with basiliximab offers no added advantage in terms of graft survival.
To cite this abstract in AMA style:
Ajlan A, Aleid H, Ali T, Joharji H, Alabdulkarim Z, Aburiash T, Almeshari K, Nazmi A, Shah Y, Devol E, Alkortas D, Broering D, Alahmadi I, Ullah A, Alotaibi A, Aljedai A. Standard Induction with Basiliximab versus no Induction in Low Immunological Risk Kidney Transplant Recipients: Study Protocol for a Randomized Controlled Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/standard-induction-with-basiliximab-versus-no-induction-in-low-immunological-risk-kidney-transplant-recipients-study-protocol-for-a-randomized-controlled-trial/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress