A Sign of Things to Come? Clinical Markers After Elevated Donor-Derived Cell-Free DNA in Heart Transplant Recipients
1Cardiology, Stanford University, Stanford, CA, 2Transplant, Stanford Health Care, Stanford, CA, 3Pathology, Stanford University, Stanford, CA, 4Cardiology, University Health Network, Toronto, ON, Canada, 5Cardiology, University of Chicago, Chicago, IL
Meeting: 2022 American Transplant Congress
Abstract number: 237
Keywords: Graft function, Heart transplant patients, Non-invasive diagnosis, Rejection
Topic: Clinical Science » Heart » 63 - Heart and VADs: All Topics
Session Information
Session Name: Heart and VADs: All Topics II
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:00pm-4:10pm
Presentation Time: 4:00pm-4:10pm
Location: Hynes Room 210
*Purpose: Endomyocardial biopsy (EMBx) has historically been the standard of care for surveillance post heart transplant (HTx), but is limited by sample area and interpretation. Donor-derived cell free DNA (ddcfDNA) testing serves as non-invasive rejection surveillance and assesses for damage throughout the allograft. This study considered the relation of elevated ddcfDNA with the incidence of four clinical markers for up to 6 months following the elevation.
*Methods: This single center study included adult heart transplant recipients from January 2019 to March 2021 with at least one ddcfDNA result ≥0.12%. Multiorgan transplant recipients and patients transitioned to other transplant centers for follow-up were excluded. This study considered the following markers: acute cellular rejection (ACR), antibody-mediated rejection (AMR), ejection fraction (EF) <50%, and de novo donor specific antibodies (DSA).
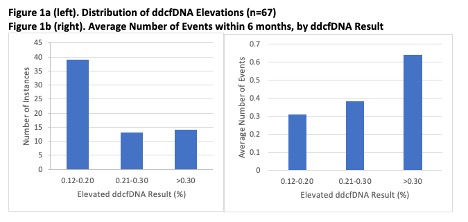
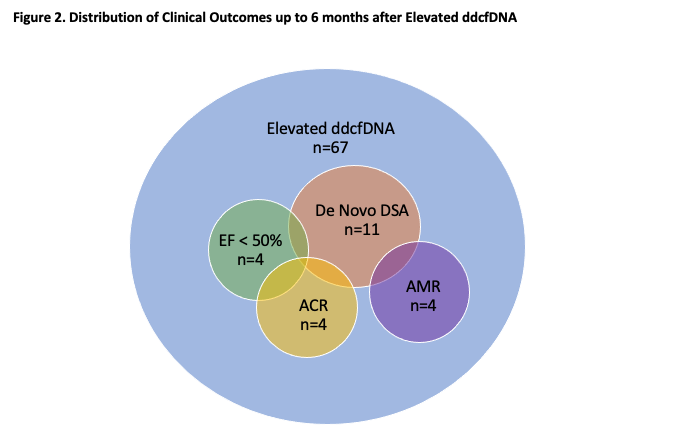
*Results: In total, 67 elevated ddcfDNA results from 56 patients (mean 0.24%, median 0.19%, distribution shown in Figure 1a) were studied. 4 ACR, 4 AMR, 4 EF <50%, and 11 de novo DSA episodes were observed. There were 2 episodes of ACR and de novo DSA, 2 of AMR and de novo DSA, and 1 of EF <50%, ACR, and de novo DSA. 46 elevations were associated with no event (Figure 2). Figure 1b shows the average number of events experienced, grouped by ddcfDNA result. Results between 0.12-0.20% corresponded with an average of 0.31 events in the following 6 months, results between 0.21-0.30% preceded an average of 0.38, and results >0.30% preceded an average of 0.64.
*Conclusions: Currently, ddcfDNA serves as non-invasive rejection surveillance, but this data demonstrates that elevations may presage future risk for not only rejection, but also graft dysfunction and de novo DSA. The data also suggests that higher ddcfDNA results, especially those >0.30%, may be associated with greater likelihoods of negative clinical outcomes in the 6 months following the result.
To cite this abstract in AMA style:
Kim DT, Henricksen EJ, Zhang BM, Teuteberg JJ, Moayedi Y, Luikart HI, Han J, Wayda B, Khush K. A Sign of Things to Come? Clinical Markers After Elevated Donor-Derived Cell-Free DNA in Heart Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/a-sign-of-things-to-come-clinical-markers-after-elevated-donor-derived-cell-free-dna-in-heart-transplant-recipients/. Accessed February 9, 2026.« Back to 2022 American Transplant Congress