Impact of Immunosuppression on T-cell Responses After mRNA-Based Sars-CoV-2 Vaccination
E. Wong1, N. L. Bert2, H. K. Sran1, A. Lim1, M. D'Costa1, K. Akalya2, S. Hang2, A. Bertoletti2, A. Vathsala1
1National University Centre for Organ Transplantation, National University Hospital, Singapore, Singapore, 2Programme in Emerging Infectious Diseases, Duke-National University of Singapore Medical School, Singapore, Singapore
Meeting: 2022 American Transplant Congress
Abstract number: 977
Keywords: COVID-19, T cells, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Administration of mRNA-based SARS-CoV-2 vaccines confers protection from SARS-CoV-2 infection and reduces its severity in the general population. It has been suggested that mounting a coordinated adaptive immune response characterized by production of neutralizing antibodies and SARS-CoV-2 spike protein-specific T-cells correlates with protection from infection. Studies in organ transplant recipients have demonstrated suboptimal responses after 2 doses of SARS-CoV-2 vaccination; however, the impact of different immunosuppressive regimens (IS) on T-cell responses is not well described. This study prospectively evaluated the impact of IS on T-cell responses in a kidney transplant (KTx) population and compared these to 26 healthy controls.
*Methods: In this single-centre, prospective study, 92 KTx on follow-up at our centre were enrolled after informed consent. T-cell responses were evaluated before and after each of 2 doses of BNT162b2 SARS-CoV-2 vaccine administered 21 days apart: before each dose, 10-14 days after Dose1 and 21-24 days after Dose2. The study population included 69.6% Live-Donor and 30.4% Deceased-Donor KTx. Longitudinal assessment of the quantity of spike-specific T-cells was performed by stimulating whole blood with peptides covering the SARS-CoV-2 spike protein, followed by cytokine (IFN-γ, IL-2) measurement (JCI, Tan et al, 2021). KTx were stratified by maintenance IS into 4 groups and T-cell responses compared between groups.
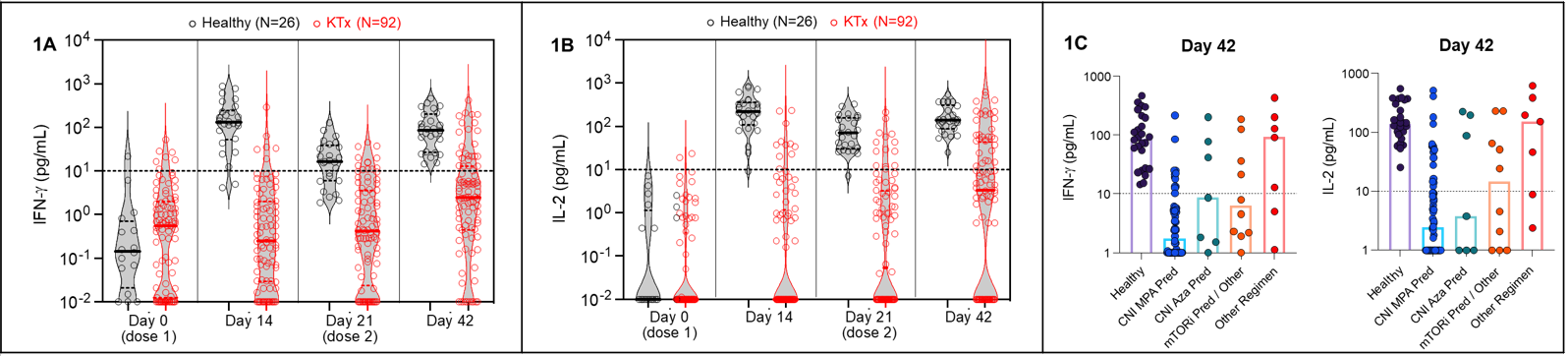
*Results: As shown (Figures 1A, 1B), in comparison to healthy controls, KTx displayed poor spike-specific T-cell responses as measured by IFN-γ and IL-2 release. Percent responders were significantly lower for KTx vs. healthy controls: 6.5% vs. 92.3% after Dose1 (P<0.00001) and 27.2% vs. 100% after Dose2 respectively. There was a significant impact of different IS regimens (Figure 1C); percent responders after Dose2 were 19%, 43%, 40% and 71% for KTx receiving CNI_MPA_Pred, CNI_Aza_Pred, mTORi and Other regimens respectively (P=0.013).
*Conclusions: Our results highlight the critical role of IS on T-cell responses to SARS-CoV-2 vaccination. In the context of the COVID-19 pandemic, monitoring T-cell and antibody responses over time after vaccination, modulating IS and modifying vaccination strategies are clearly needed to protect this vulnerable population.
To cite this abstract in AMA style:
Wong E, Bert NL, Sran HK, Lim A, D'Costa M, Akalya K, Hang S, Bertoletti A, Vathsala A. Impact of Immunosuppression on T-cell Responses After mRNA-Based Sars-CoV-2 Vaccination [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-immunosuppression-on-t-cell-responses-after-mrna-based-sars-cov-2-vaccination/. Accessed February 6, 2026.« Back to 2022 American Transplant Congress