Humoral and Cellular Immune Response to a Third Dose Of SARS-CoV-2 Vaccine in Kidney Transplant Recipients on Belatacept
J. Mitchell1, J. Alejo1, T. P. Chiang1, J. N. Blankson1, A. H. Karaba1, B. Woldemeskel1, C. Garliss1, A. Chang1, A. Abedon1, R. K. Avery1, A. Tobian1, A. B. Massie1, J. Garonzik-Wang2, D. L. Segev1, W. Werbel1
1Johns Hopkins University, Baltimore, MD, 2University of Wisconsin, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 969
Keywords: COVID-19, Immunogenicity, Immunosuppression, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
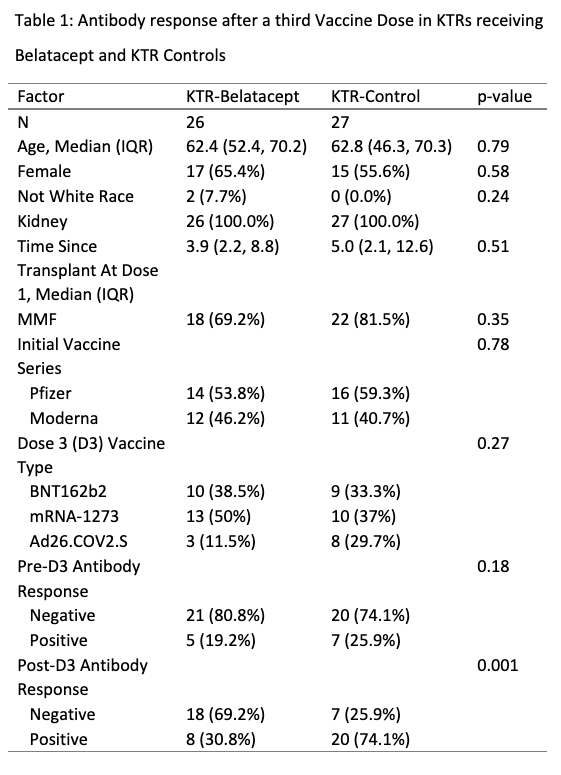
*Purpose: Kidney transplant recipients taking belatacept (KTR-B) have poor immune response to two-dose SARS-CoV-2 vaccination. We sought to characterize the impact of an additional vaccine dose on plasma neutralizing capacity and cellular responses as compared to that of KTRs controls (KTR-C) not taking belatacept.
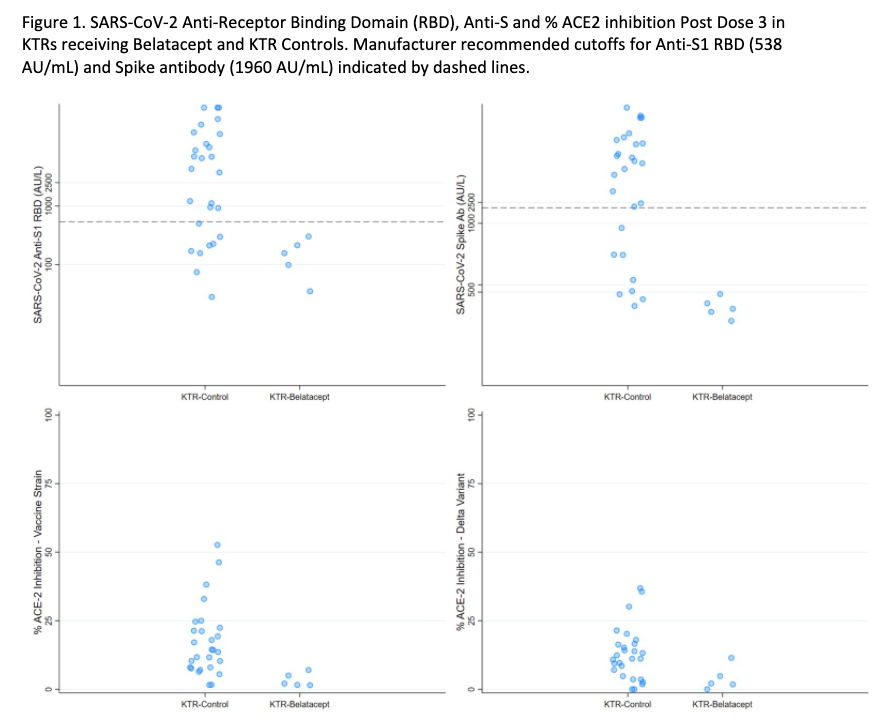
*Methods: Within an observational cohort, we tested 26 KTR-Bs and 27 KTR-Cs for anti-spike antibody responses before and after a third SARS-CoV-2 vaccine dose (D3) using two clinical assays (Roche Elecsys anti-S Ig and EUROIMMUN anti-S1 IgG). For a subset of 5 KTR-Bs and for all KTR-Cs we used a research assay (Meso Scale Diagnostics V-Plex [MSD]) to further assess anti-spike and RBD IgG, as well as surrogate plasma neutralizing activity (% ACE2 inhibition) versus the ancestral and delta variants. For 3 KTR-Bs, post D3 T cell response was assessed via IFN-y ELISpot and deemed positive if spot forming units > 20 per million PBMC and stimulation index > 3.
*Results: KTR-Bs had significant lower clinical anti-spike seroconversion than KTR-Cs (31% vs 74%, p=0.001) after D3 despite similar demographics, clinical factors, and vaccines administered (Table 1). No KTR-B (0/5) was seropositive by MSD anti-spike or anti-RBD IgG (Figure 1). % ACE2 inhibition versus the ancestral variant was significantly lower in KTR-Bs than in KTR-Cs (Median [IQR] 5.2 [2.8, 6.5] vs 12.5 [7.7, 23.9], p<0.01); all KTR-Bs were below a level consistent with detectable neutralizing antibody. All tested KTR-Bs (3/3) had a negative ELISpot, consistent with negligible cellular response.
*Conclusions: These results suggest minimal humoral or cellular immunogenicity of additional vaccine doses for KTR-Bs and indicates the need for alternative strategies to improve vaccine response such as immunosuppression alteration or use of passive immunoprophylaxis with monoclonal anti-spike antibody to improve protection versus SARS-CoV-2.
To cite this abstract in AMA style:
Mitchell J, Alejo J, Chiang TP, Blankson JN, Karaba AH, Woldemeskel B, Garliss C, Chang A, Abedon A, Avery RK, Tobian A, Massie AB, Garonzik-Wang J, Segev DL, Werbel W. Humoral and Cellular Immune Response to a Third Dose Of SARS-CoV-2 Vaccine in Kidney Transplant Recipients on Belatacept [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/humoral-and-cellular-immune-response-to-a-third-dose-of-sars-cov-2-vaccine-in-kidney-transplant-recipients-on-belatacept/. Accessed February 4, 2026.« Back to 2022 American Transplant Congress