Rhesus-Primatized Mouse Model Generated Using Transgenic Immunodeficient Mice
1Surgery, University of Wisconsin, Madison, WI, 2University of Wisconsin, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 178
Keywords: Engraftment, Mice, Primates, Stem cells
Topic: Basic Science » Basic Science » 05 - Translational Cellular Therapies: Islet and Stem Cell Transplantation
Session Information
Session Name: Cellular/Islet Therapies and Tissue Engineering
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:50pm-6:00pm
Presentation Time: 5:50pm-6:00pm
Location: Hynes Room 310
*Purpose: To develop a translational primatized mouse model, akin to humanized mice, that allows for high-throughput in vivo experimentation to augment non-human primate pre-clinical studies.
*Methods: Bead-separated CD34+ rhesus macaque cells (1×105-1×106) were intravenously transplanted into strains of immunodeficient mice crossbred with human cytokine transgenes. Autologous thymic fragments were surgically placed within the mouse kidney capsule to allow for de novo T cell development. Rhesus cross-reactive anti-CD2 was administered for passenger leukocyte depletion. Flow cytometric analyses were used for chimerism testing and immunoprofiling. Mixed lymphocyte reactions and SIVmac239 infection assays were employed for in vitro allogenic and infectious functional analysis, respectively.
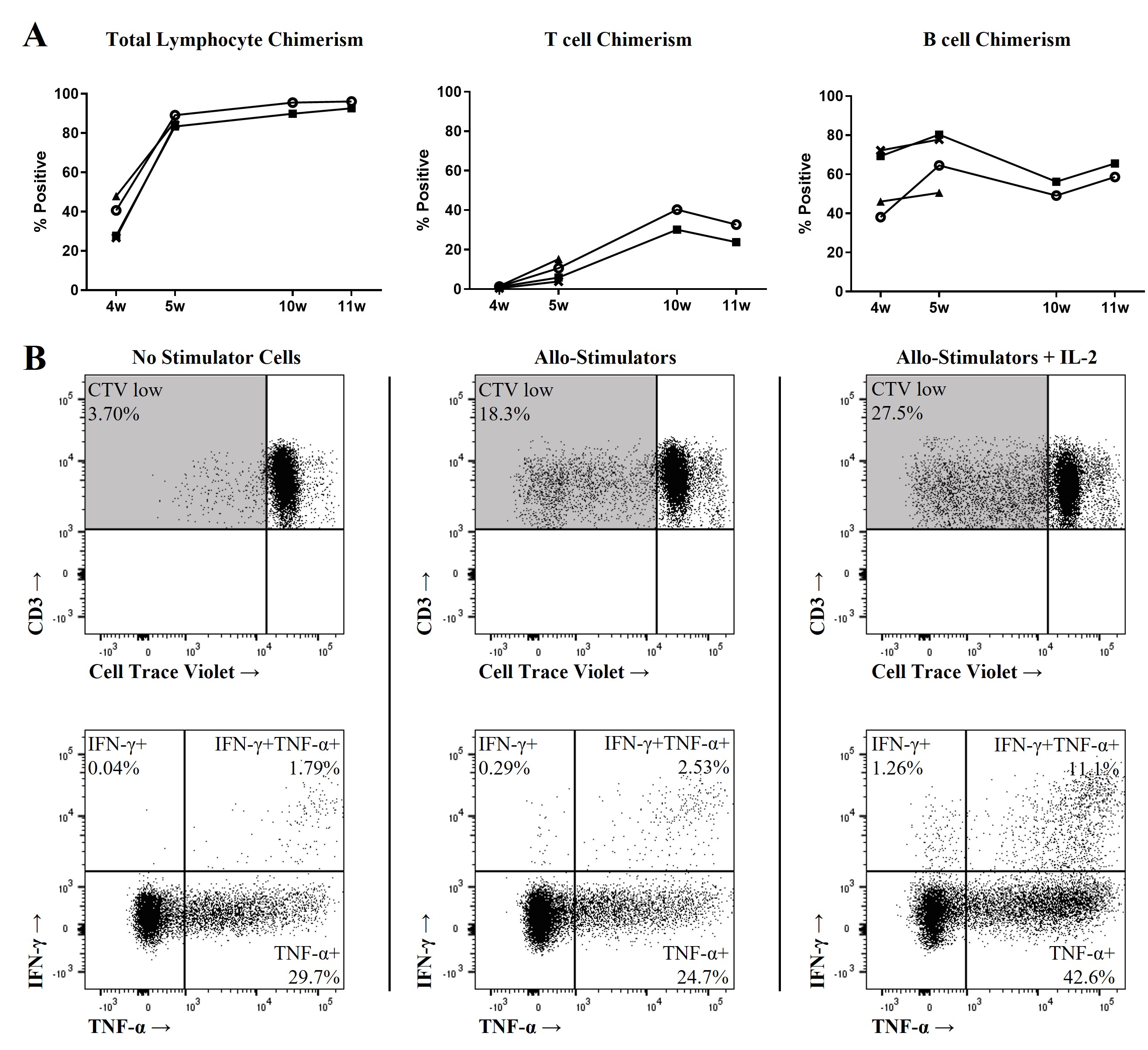
*Results: Fetal liver-derived hematopoietic stem and progenitor cells (HSPCs) were found to have higher relative CD34hi fractions (containing progenitors with the greatest engraftment potential) compared to adult mobilized blood and bone marrow. Co-transplantation of fetal liver-derived CD34-enriched HSPCs and autologous thymic fragments into immunodeficient mice harboring transgenes for human (rhesus cross-reactive) GM-CSF and IL-3 yielded robust, multilineage lymphohematopoietic engraftment (Figure 1A). The resulting emergent immune system recapitulated that of the HSPC source, with similar relative frequencies of leukocyte subsets within the thymic, secondary lymphoid, and peripheral compartments. Importantly, despite exhibiting a predominantly naive phenotype across all immune cell types, in vitro functional assays demonstrated robust cellular activation in response to non-specific and allogenic stimuli (Figure 1B). Further, primatized mouse splenocytes supported in vitro SIV infection, which yielded a robust viral replication curve.
*Conclusions: We have developed a durable primatized mouse model, characterized by robust multilineage engraftment yielding a functional macaque immune system that is well-poised for developmental and immunological studies.
To cite this abstract in AMA style:
Little C, Haynes WJ, Huang L, Daffada C, Wolfe KB, Perrin E, Simpson JA, Schmidt JAKropp, Hinkle HM, Keding LT, Behrens RT, Evans DT, Kaufman D, Thomson JA, Golos TG, Brown M. Rhesus-Primatized Mouse Model Generated Using Transgenic Immunodeficient Mice [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/rhesus-primatized-mouse-model-generated-using-transgenic-immunodeficient-mice/. Accessed January 4, 2026.« Back to 2022 American Transplant Congress