Donor-Derived IL-33 Plays a Critical Role in Lung Allograft Acceptance
1Department of Surgery, University of Maryland, Baltimore, Baltimore, MD, 2Washington University School of Medicine in St Louis, Saint Louis, MO
Meeting: 2022 American Transplant Congress
Abstract number: 185
Keywords: Eosinophils, Ischemia, Lung transplantation, Rejection
Topic: Basic Science » Basic Science » 08 - Innate Immunity; Chemokines, Cytokines, Complement
Session Information
Session Name: Innate Immunity, Chemokines, Cytokines, and Complement
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-5:40pm
Presentation Time: 5:30pm-5:40pm
Location: Hynes Room 309
*Purpose: IL-33 is considered an alarmin secreted in response to tissue damage. While it is elaborated by the lung allograft as part of ischemia-reperfusion injury its role in lung graft homeostasis is unknown. Meanwhile, it serves as regulating cytokine in both type1 and type2 immune response. Given the importance of IL-33 in local immunity, we sought to define its role in lung transplantation.
*Methods: Left single lung transplants were performed as previously described with co-stimulatory blockade (CSB) immunosuppression and evaluated at day 7. A hilar clamping model of ischemia-reperfusion injury (IRI) was used to confirm some results.
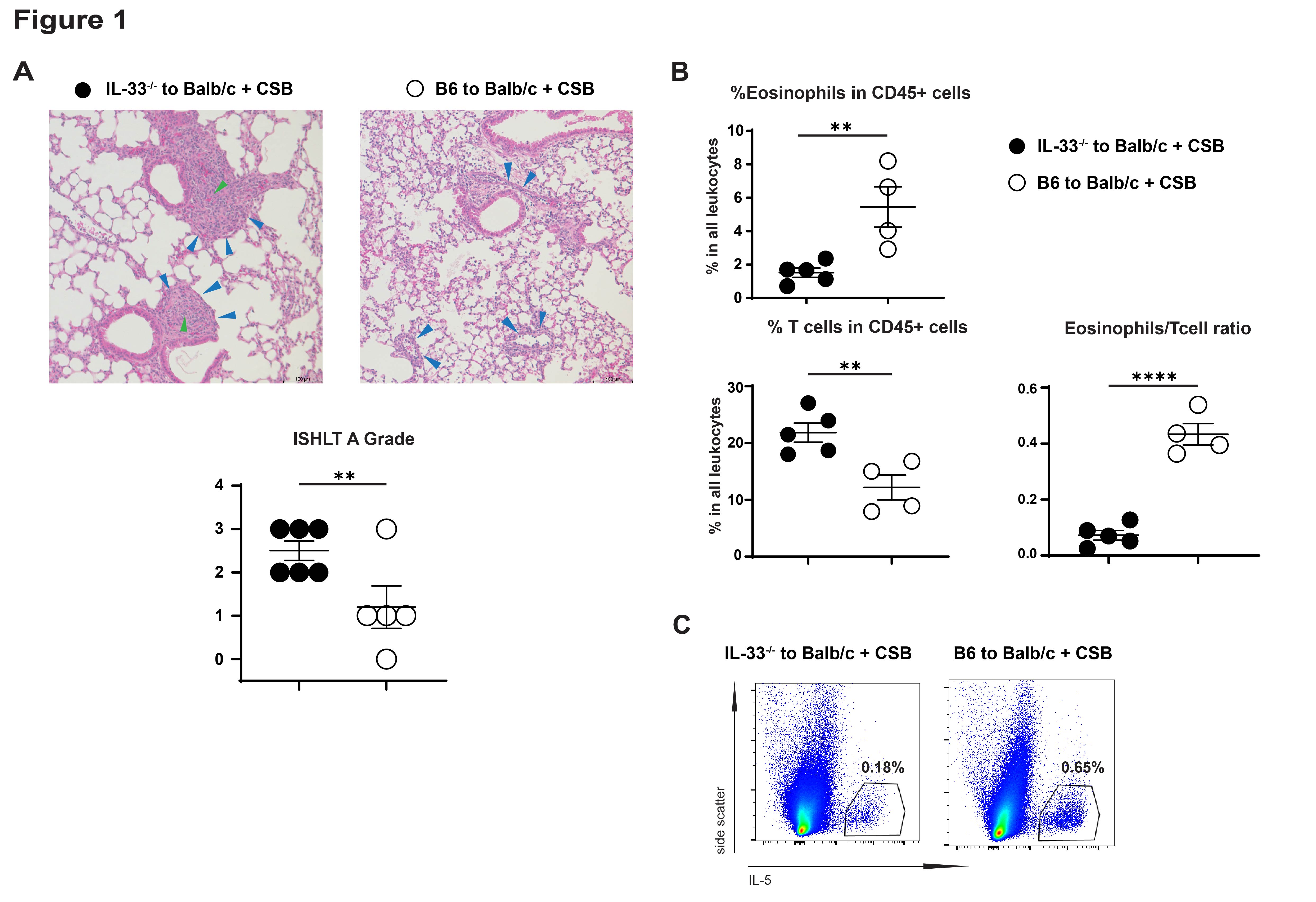
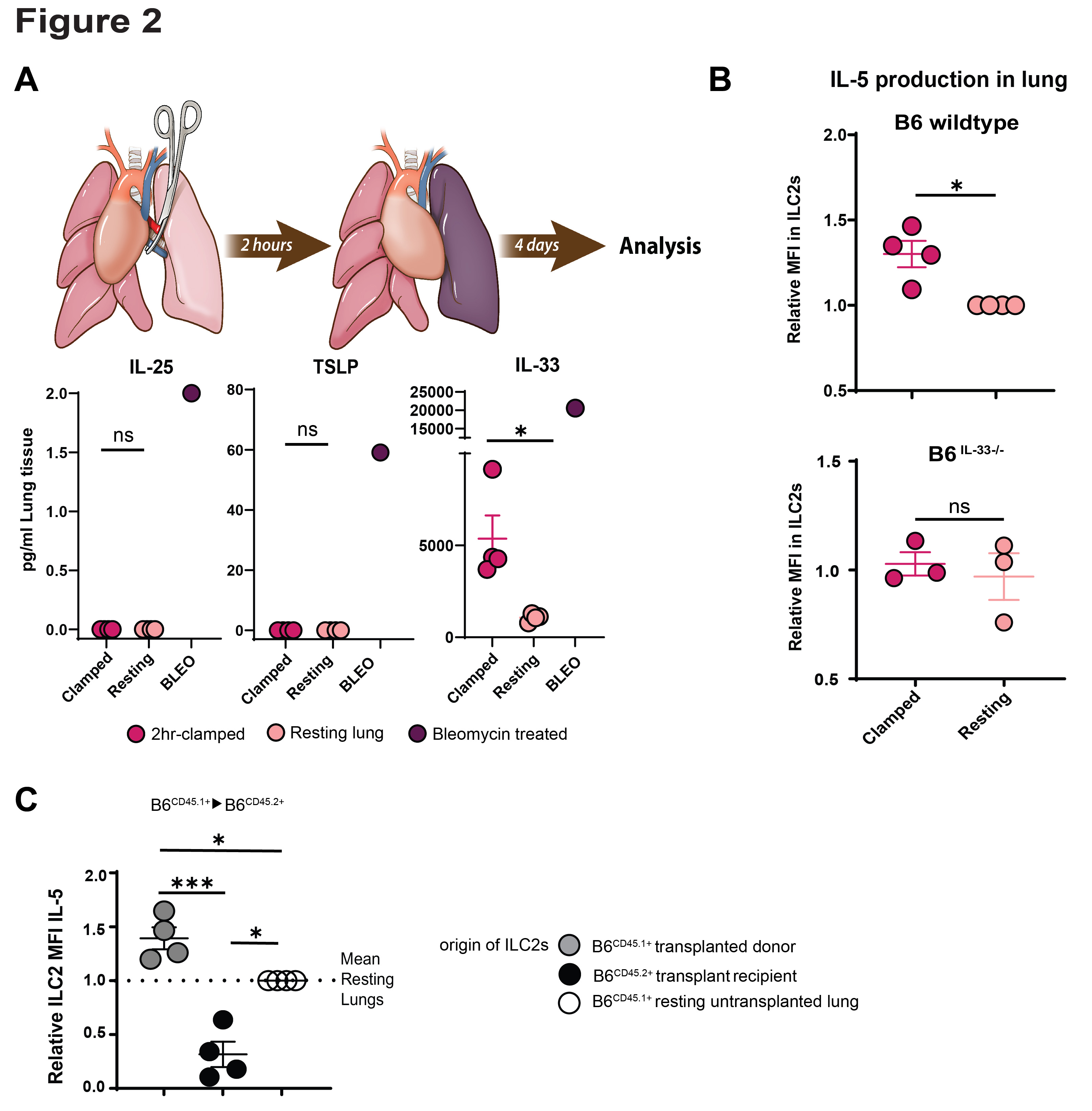
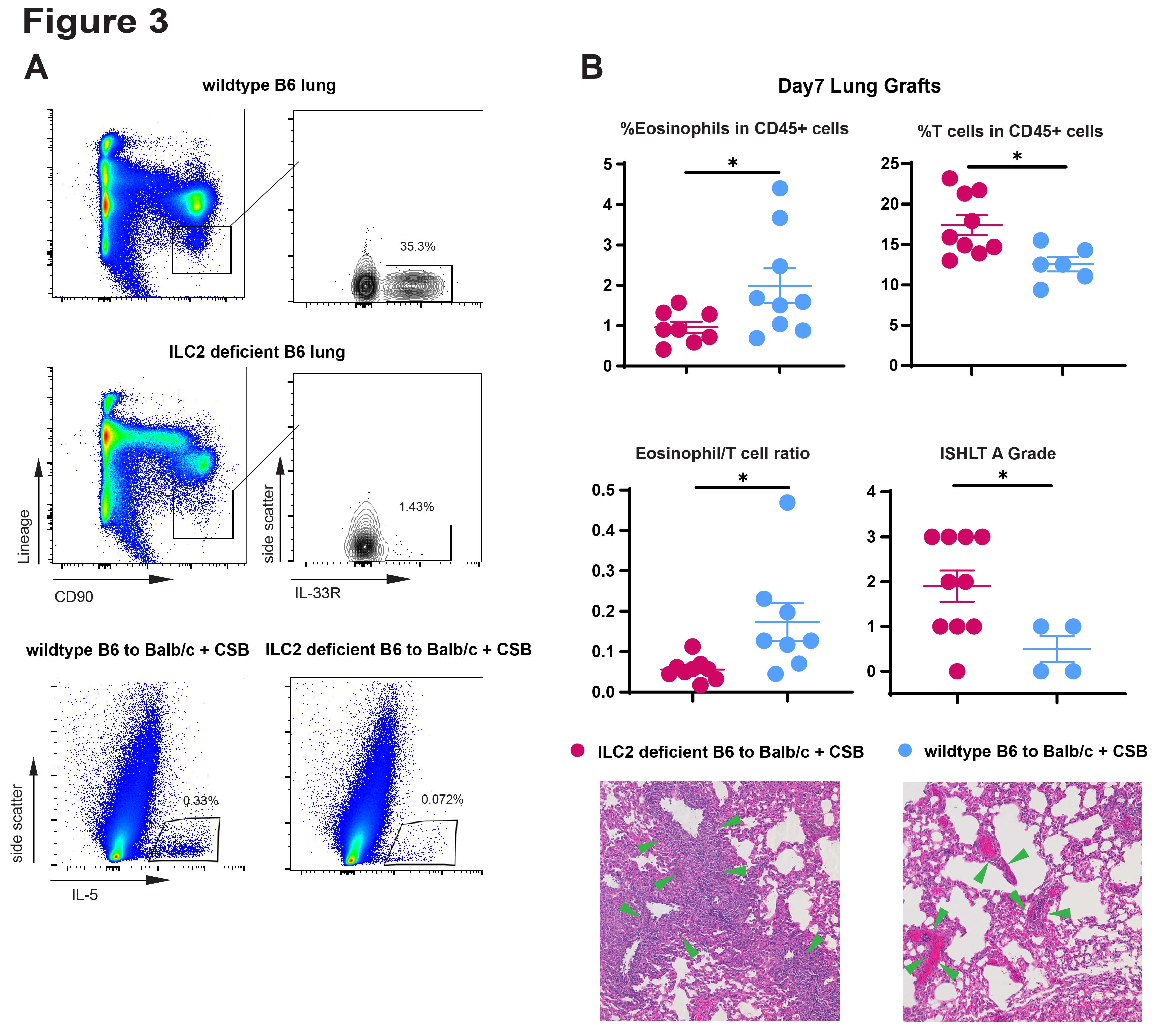
*Results: IL-33 deficient lung allografts on a C57BL/6 background transplanted into Balb/c mice were rejected despite CSB immunosuppression. This was characterized by an increase in the ISHLT rejection grades, fewer tolerogenic eosinophils and more T cells, and lower production of IL-5 (Figure 1). Thus IL-33 plays a role in lung allograft acceptance. Using the hilar clamp model of IRI we were able to prove that IL-33 levels depended on ischemic injury and that IL-5 secretion by ILC2 depended on IL-33 (Figure 2). ILC2 deficient lung allografts demonstrated low IL-5 production after transplantation and a similar rejection pattern observed on IL-33 deficient lung allografts (Figure 3).
*Conclusions: Our data expands on our previous observations that eosinophil homeostasis and migration to the lung graft, which depends on IL-5, is critical to tolerance induction. We now demonstrate that IL-33 induced by IRI activates donor-derived ILC2s which secrete IL-5 into the local environment to facilitate eosinophil-mediated tolerance. Taken together, donor-derived IL-33 initiates a protective mechanism against allograft rejection.
To cite this abstract in AMA style:
Guo Y, Mei Z, Khalil M, Li D, Banerjee A, Gelman A, Kreisel D, Krupnick A. Donor-Derived IL-33 Plays a Critical Role in Lung Allograft Acceptance [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-derived-il-33-plays-a-critical-role-in-lung-allograft-acceptance/. Accessed March 4, 2026.« Back to 2022 American Transplant Congress