Poor Tolerability of CFTR Modulator Therapy in Lung Transplant Recipients
UNC Health, Chapel Hill, NC
Meeting: 2022 American Transplant Congress
Abstract number: 157
Keywords: Efficacy, Lung transplantation, Post-operative complications, Toxocity
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Name: Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:50pm-7:00pm
Presentation Time: 6:50pm-7:00pm
Location: Hynes Room 312
*Purpose: Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) is a highly effective therapy for cystic fibrosis (CF) with potential benefits in lung transplant recipients (LTRs) for extrapulmonary CF manifestations, however tolerability and efficacy in this population is largely unknown.
*Methods: All LTR at a single center initiated on ELX/TEZ/IVA were reviewed. Adverse events and patient reported outcomes attributed to ELX/TEZ/IVA were documented. Pulmonary function, tacrolimus requirements in mg/kg/dL, body mass index (BMI), and reason for initiation of therapy were assessed at the initiation, and at 12 months post-initiation or at the time of discontinuation for those in whom therapy was discontinued.
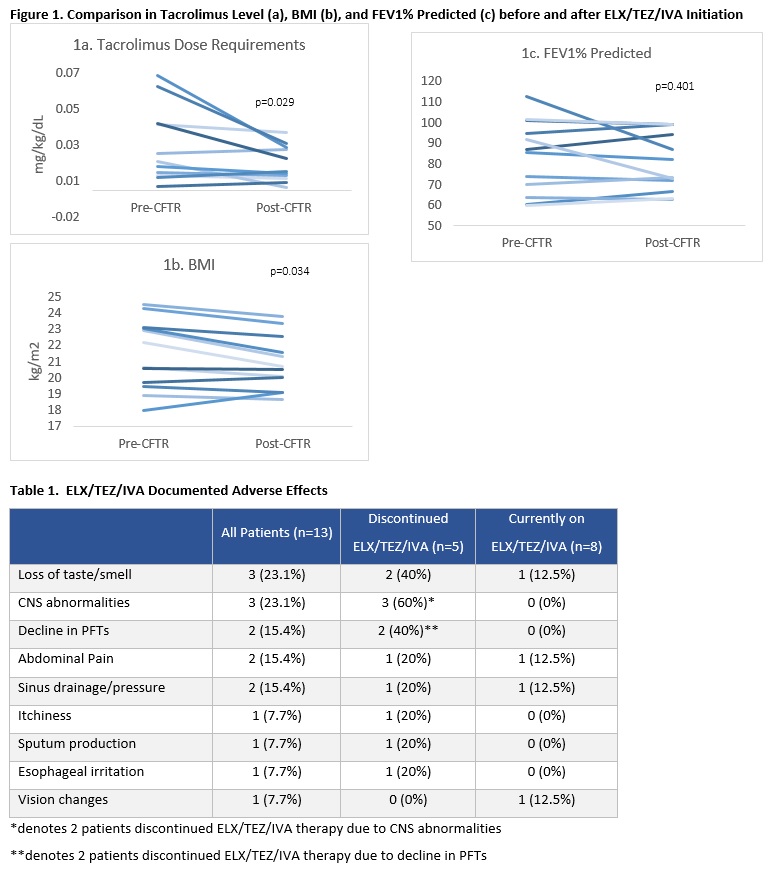
*Results: 13 LTRs were initiated on ELX/TEZ/IVA at 115 ± 92 months post-transplant. 9 (69.2%) were male and all were Caucasian. The mean age was 36.8 ± 12.6. 7/13 (53.8%) initiated ELX/TEZ/IVA for sinus disease; 6/13 (46.2%) initiated therapy for sinus and GI disease. 5/13 (38.4%) discontinued therapy at a median of 195d following initiation (range 47-498 days); two (40%) for decline in PFTs, two (40%) for mood disturbances, and one (20%) for lack of perceived benefit with ELX/TEZ/IVA therapy. In both LTRs for whom ELX/TEZ/IVA was discontinued due to PFT decline, PFTs returned to baseline following cessation of therapy. One of the two LTR who discontinued ELX/TEZ/IVA for PFT decline re-challenged therapy 12 months later, with a repeated decline in PFTs and repeat return to baseline following discontinuation. Similarly, both LTR who discontinued for mood disturbances reported return to baseline following discontinuation. No change in percent predicted FEV1 was seen in the cohort as a whole (Figure 1c). Of the 8 LTRs who remain on ELX/TEZ/IVA, four reported adverse effects and three LTRs temporarily held therapy (Table 1). Six (46.2%) LTRs reported improvement in sinus symptoms, while four (30.7%) reported improved gastrointestinal symptoms. Only 1 LTR reported improvements in glycemic control or weight gain. Weight as assessed by BMI declined in the cohort overall, with BMI at the time of ELX/TEZ/IVA initiation higher than at either discontinuation or 12 months post-initiation (21.5 vs. 20.6, p=0.034) (Figure 1b). Tacrolimus dose requirements decreased following initiation of ELX/TEZ/IVA therapy, with a 50% decline in dose requirements from 0.02 mg/kg/dL to 0.01 mg/kg/dL, p=0.029 (Figure 1a).
*Conclusions: In a single center experience, ELX/TEZ/IVA in LTRs is poorly tolerated with modest perceived extrapulmonary benefit and a significant effect on tacrolimus dose requirements. More data is needed to determine the benefits of ELX/TEZ/IVA therapy in LTRs.
To cite this abstract in AMA style:
Doligalski C, McKinzie C, Yang A, Coakley R. Poor Tolerability of CFTR Modulator Therapy in Lung Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/poor-tolerability-of-cftr-modulator-therapy-in-lung-transplant-recipients/. Accessed March 6, 2026.« Back to 2022 American Transplant Congress