Safety and Efficacy of Peri-Vaccination Antimetabolite Hold to Augment Third SARS-CoV-2 Vaccine Dose Response in Lung Transplant Recipients
Johns Hopkins University School of Medicine, Baltimore, MD
Meeting: 2022 American Transplant Congress
Abstract number: 43
Keywords: COVID-19, Efficacy, Lung transplantation, Safety
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Name: Infectious Considerations for Lung Transplantation
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:50pm-4:00pm
Presentation Time: 3:50pm-4:00pm
Location: Hynes Room 210
*Purpose: Lung transplant recipients (LTRs) are less likely than other solid organ transplant recipients (SOTRs) to develop an antibody response to SARS-CoV-2 vaccination. Mycophenolate has been associated with impaired humoral response to SARS-CoV-2 vaccination, leaving these patients vulnerable to infection. Peri-vaccination antimetabolite hold has been an effective strategy for some patients with rheumatic and musculoskeletal diseases. In this study, we describe the safety and efficacy of a peri-vaccination antimetabolite hold in LTRs on SARS-CoV-2 anti-spike protein development following a third vaccine dose.
*Methods: We studied LTRs on mycophenolate or azathioprine based antimetabolite therapy (AMT) >1-year post-LT with no history of acute cellular or humoral rejection who received SARS-CoV-2 vaccine Dose 3 (D3) May-October 2021. Patients were instructed to hold AMT 1 week before and 2 weeks after D3. Antibody titers were measured pre- and post-D3 (Roche Elecsys or DiaSorin Liaison anti-S EIA). Safety was evaluated using donor specific antibody (DSA) trend and lung biopsy results.
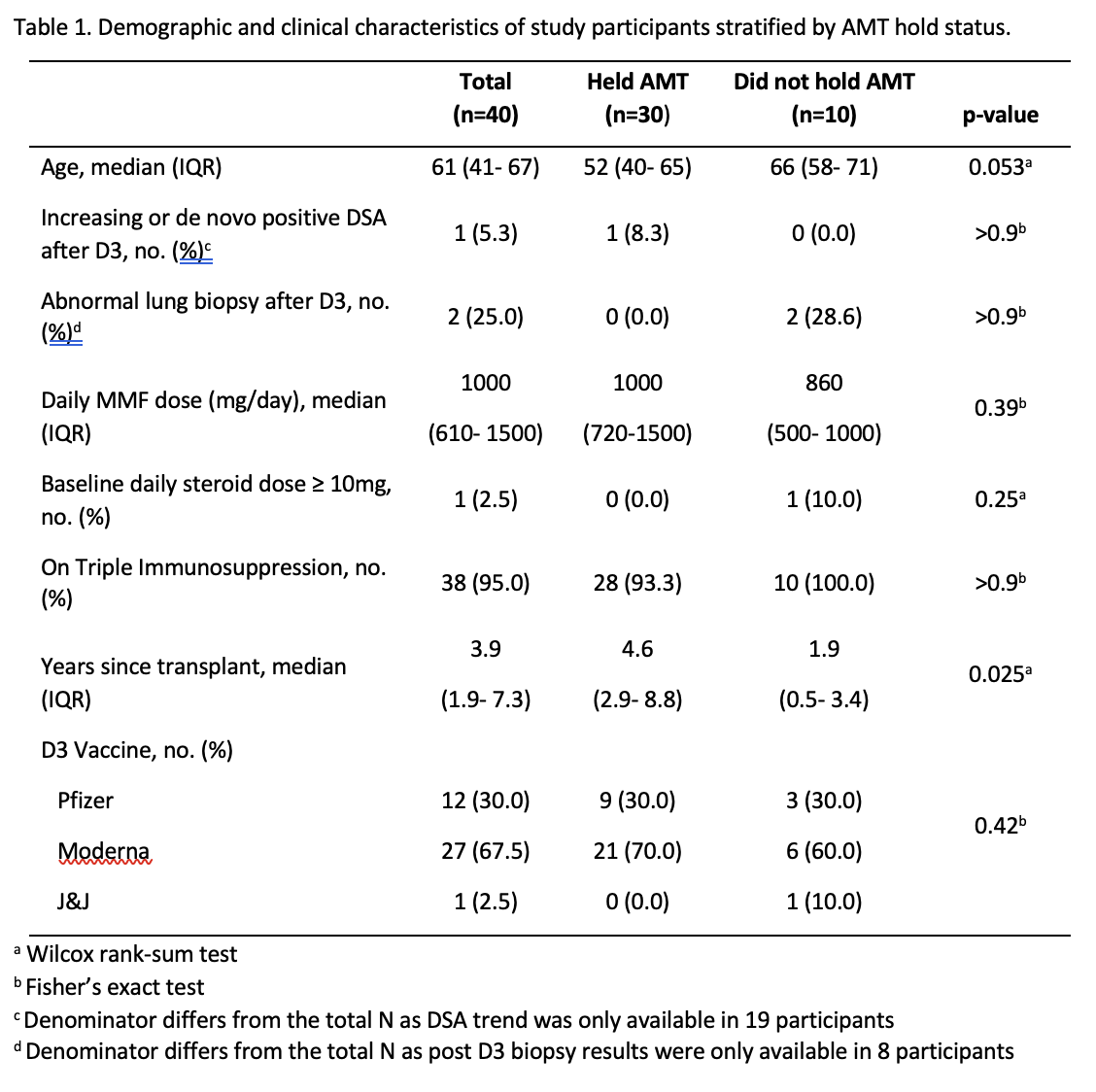
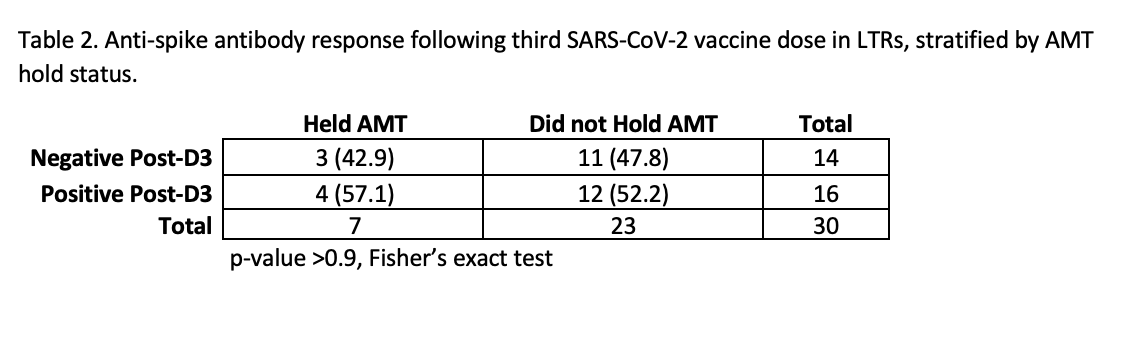
*Results: Of 40 participants, 30 held AMT and 10 did not hold AMT. Median (IQR) time since transplant was 3.9 years (1.9-7.3). Median (IQR) time between vaccine dose 2 and D3 was 163 days (143- 180). Among participants seronegative pre-D3, seroconversion post-D3 occurred in 52% (12/23) who held AMT vs. 57% (4/7) who did not hold AMT (p>0.9). Among patients with pre- and post-D3 DSA levels (N=19), increasing or de novo positive DSA was seen in 8.3% (1/12) of patients who held AMT vs. 0% of patients who did not hold AMT. For patients who underwent lung biopsy post-D3 (N=8), abnormal biopsy was seen in 0% (0/1) of patients holding AMT vs. 28.6% (2/7) not holding AMT.
*Conclusions: A three-week AMT hold by LTRs was not associated with increased rejection but also showed no significant difference in seroconversion compared to LTRs who did not hold AMT, suggesting this hold protocol is safe but not effective. Further investigation of alternative strategies is needed to protect LTRs from COVID-19 infection who do not mount effective immunoprotection following SARS COV-2 vaccination.
To cite this abstract in AMA style:
Frey S, Ruck J, Alejo J, Barker L, Werbel W, Avery R, Segev D, Shah P. Safety and Efficacy of Peri-Vaccination Antimetabolite Hold to Augment Third SARS-CoV-2 Vaccine Dose Response in Lung Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-peri-vaccination-antimetabolite-hold-to-augment-third-sars-cov-2-vaccine-dose-response-in-lung-transplant-recipients/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress