Humoral Response After a Third Dose of Sars-CoV-2 mRNA Vaccine in Transplant Recipients
Brigham & Women's Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 42
Keywords: COVID-19, Heart/lung transplantation, Kidney transplantation, Vaccination
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Name: Infectious Considerations for Lung Transplantation
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:40pm-3:50pm
Presentation Time: 3:40pm-3:50pm
Location: Hynes Room 210
*Purpose: Prior studies demonstrated an increased immune response post-3rd COVID-19 vaccine dose when given 1-month post-2nd dose in solid organ transplant recipients (SOT). This study assessed whether a 3rd mRNA vaccine dose administered 6 months post-2nd dose enhanced humoral immune response in SOT.
*Methods: A prospective cohort study was conducted of SOT, without prior COVID-19, who received 3 mRNA vaccine doses (BNT162b2 (Pfizer) or mRNA-1273 (Moderna)). Primary outcome was a positive serologic response characterized by an anti-receptor-binding domain (RBD) antibody (Ab) level of >100 U/mL 4 weeks post-3rd dose (measured with the Roche Elecsys anti-SARS-CoV-2 immunoassay).
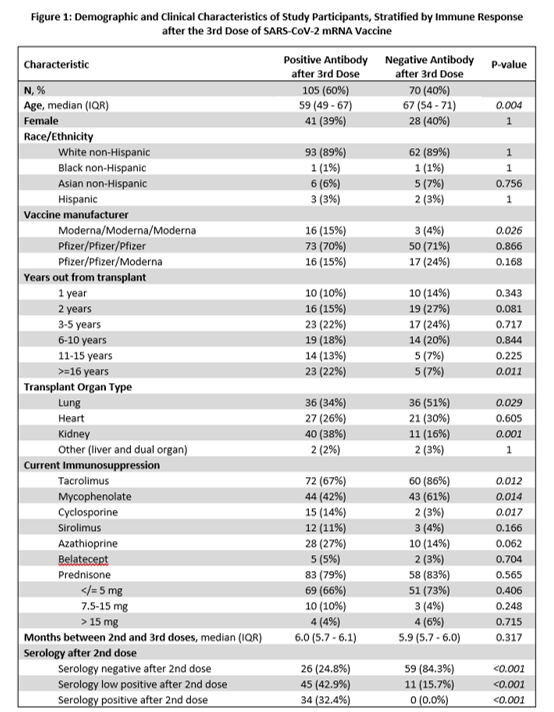
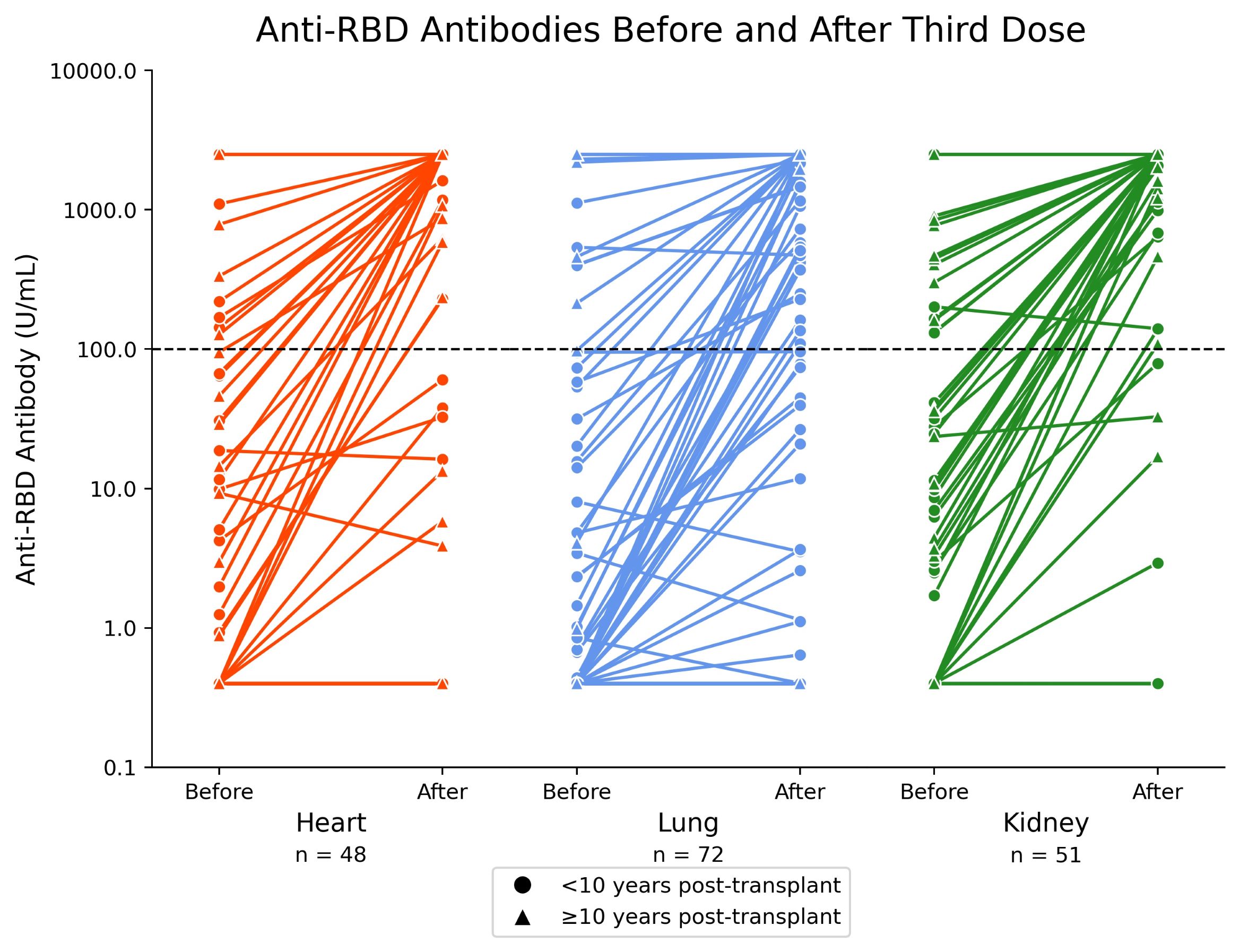
*Results: 175 SOT were enrolled: 48 (27%) heart, 72 (41%) lung, 51 (29%) kidney, 4 (2%) other SOT (Figure 1). A positive anti-RBD Ab level measured 4-weeks post-3rd vaccine dose was present in 105/175 (60%). In a multivariable model, age >60 years (adjusted odds ratio [aOR] 0.41; 95% CI 0.19 to 0.87), heart (aOR 0.28; 95% CI 0.10 to 0.76) or lung (aOR 0.20; 95% CI 0.07 to 0.55) transplant, and mycophenolate use (aOR 0.24; 95% CI 0.11 to 0.54) were independent risk factors for not developing a positive Ab post-3rd dose. Patients transplanted >10 years ago were more likely to have a positive Ab (aOR 4.95; 95% CI 1.04 to 23.42). Those with a low positive Ab (>0.8 to <100 U/mL) post-2nd vaccine dose were more likely to have a positive Ab post-3rd dose than those with an undetectable Ab (<0.8 U/mL). But 26/105 (25%) participants with an undetectable Ab post-2nd dose developed an Ab of >100 U/mL; 9/26 (35%) developed an Ab >1000 U/mL (Figure 2). 4 (2%) SOT developed rejection within 30-days post-3rd dose.
*Conclusions: Despite the extended time interval of 6 months between the 2nd and 3rd doses, the proportion of non-responders (40%) in this study was similar to other studies when the 3rd dose was given 1-month post-2nd dose. These data suggest that a substantial proportion of SOT remain at risk for COVID-19 after 3 mRNA vaccine doses. Additional interventions need to be further studied, including monoclonal Ab as pre-exposure prophylaxis and 4th vaccine doses.
To cite this abstract in AMA style:
Woolley AE, Sharma N, Zhou G, Kim A, Ryan E, Joyce M, Goldberg H, Givertz M, Mehra M, Woodcome E, Durney V, Marshall S, Townsend K, Coppolino A, Tullius S, Malek S, Mallidi H, Baden L, Chandraker A. Humoral Response After a Third Dose of Sars-CoV-2 mRNA Vaccine in Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/humoral-response-after-a-third-dose-of-sars-cov-2-mrna-vaccine-in-transplant-recipients/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress