DCD Liver Grafts Can Safely be Used for Recipients with Grade 1-2 Portal Vein Thrombosis
1Liver Transplant, Mayo Clinic, Jacksonville, FL, 2Transplant, Mayo Clinic, Jacksonville, FL, 3Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, 4Transplant Surgery, Mayo Clinic, Jacksonville, FL, 5Transplant Surgery, Mayo Clinic, Rochester, MN, 6Radiology, Mayo Clinic, Jacksonville, FL
Meeting: 2022 American Transplant Congress
Abstract number: 867
Keywords: Donation, Graft survival, Liver cirrhosis, Liver transplantation
Topic: Clinical Science » Liver » 55 - Liver: Recipient Selection
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Limited data is available in the literature regarding patients with portal vein thrombosis (PVT) receiving a liver transplant (LT) from a donation after circulatory death (DCD) donor. PVT is a common complication associated with cirrhosis. Considering that experienced transplant centers have demonstrated that better patient outcome rates and graft survival rates can be achieved with careful donor-recipient selection, as well as equivalent results between DCD LTs and DBD (Donation after brain death) LTs, more data is needed for PVT and DCD liver transplantation. This may promote expansion of DCD LTs after a careful recipient-donor selection.
*Methods: All DCD LT performed at Mayo Clinic-Florida and Mayo Clinic-Rochester from 2006-2020 were reviewed (N= 489). Patients with PVT were graded using Yerdel classification (Grade 1 – partially thrombosed PV, Grade 2 – occlusion of PV with or without minimal extension into SMV, Grade 3-4 – complete occlusion of PV with extension into SMV). A 1:3 propensity match between patients with PVT and those without PVT was performed.
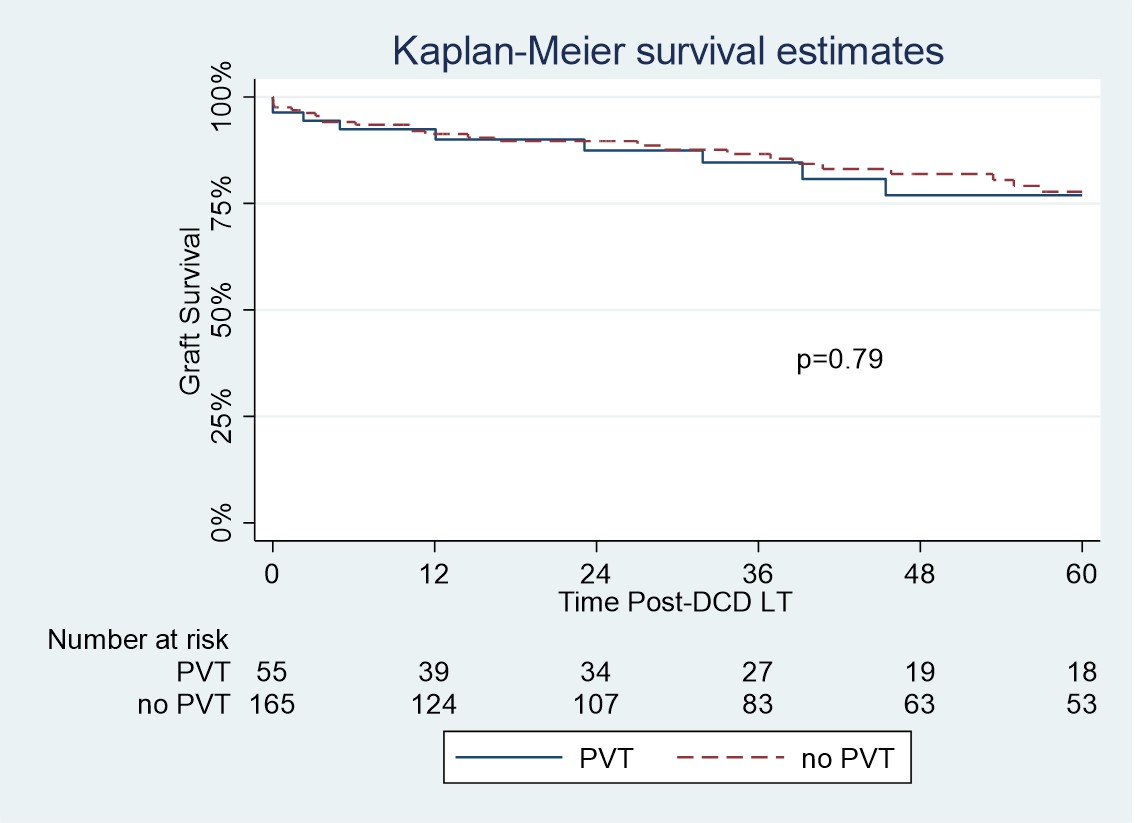
*Results: A total of 55 (9.7%) patients with PVT undergoing DCD LT were identified. Grade 1 PVT was present in 63% of patients, Grade 2 PVT in 32% and Grade 3-4 in 3%. Preoperative anticoagulation treatment was received by 34.5% of patients, 23.6% received warfarin, 7.3% enoxaparin and 3.6% apixaban. Following propensity matching there were no significant difference in donor and recipient characteristics between the PVT and no-PVT groups. Graft survival and patient survival was similar between the 2 groups. Graft survival was 89.9%, 84.5% and 76.8% at 1, 3 and 5 years respectively in the PVT group (p=0.79). The rate of any grade of ischemic cholangiopathy was 9.0% in the PVT group and 7.3% in the no-PVT group (p=0.66). There was also no significant difference in the rates of early allograft dysfunction (33.9% vs 36.3%; p=0.75) or primary non-function (0% vs 1.3%; p=0.40) between the PVT and no-PVT groups respectively. Mean number units of RBCs were 8.4 vs 7.4; p=0.32 and FFP 8.5 vs 5.9; p=0.01 in the PVT and no-PVT groups respectively.
*Conclusions: In appropriately selected recipients with Grade1-2 PVT, DCD allografts can safely be utilized with excellent outcomes.
To cite this abstract in AMA style:
Mercado LA, Bhangu HK, Musto KR, Watt KD, Taner B, Rosen CB, Taner T, Yang L, Harnois DM, LeGout JD, Croome KP. DCD Liver Grafts Can Safely be Used for Recipients with Grade 1-2 Portal Vein Thrombosis [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/dcd-liver-grafts-can-safely-be-used-for-recipients-with-grade-1-2-portal-vein-thrombosis/. Accessed February 26, 2026.« Back to 2022 American Transplant Congress