Non-Chimeric HLA-Identical Renal Transplant Tolerance: Regulatory Immunophenotypic/Genomic Biomarkers
1CTC, Northwestern University, Chicago, IL
2Department of Molecular and Experimental Medicine, Scripps Research Institute, La Jolla, CA.
Meeting: 2015 American Transplant Congress
Abstract number: 235
Keywords: Genomic markers, Kidney transplantation, Stem cells, Tolerance
Session Information
Session Name: Concurrent Session: Tolerance: Clinical Studies
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:27pm-2:39pm
Presentation Time: 2:27pm-2:39pm
Location: Room 122-AB
Background. We previously described 3-year results of a non-chimeric tolerance protocol in HLA-identical living donor renal transplant (RT) recipients and now report on longer term, more definitive findings.
Methods. Recipients with alemtuzumab induction and tacrolimus/mycophenolic acid therapy were multiply infused with donor hematopoietic CD34+ stem cells (DHSC) following early conversion to sirolimus. All immunosuppression was withdrawn by 24 months. Twelve months later tolerance was documented by rejection-free transplant biopsies.
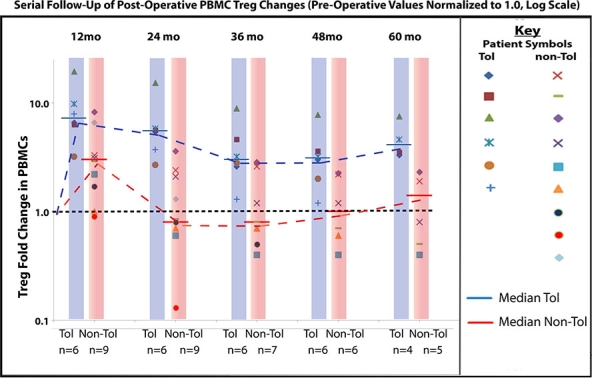
Results. Five of the first 8 enrollees were initially shown as tolerant at 3 years, 1-year off immunosuppression. Biopsies of 3 patients after total withdrawal showed Banff 1A acute cellular rejection without renal dysfunction. With longer follow-up of the 8 original patients including 5-year post-transplant biopsies (5 to 6.5 years), 4 of the 5 tolerant recipients remain without rejection evidence while one revealed Banff 1A rejection at 5 years without renal dysfunction. By including the next 7 more newly entered subjects (2 tolerant at 3 years), we demonstrate sequentially elevated numbers of circulating CD4+CD25+++CD127–FOXP3+ Tregs in tolerant subjects vs. a loss of Tregs in non-tolerant subjects (see figure, p≤0.001), with concrete evidence of DHSC dose-related immunoregulation in tolerant patients. We also demonstrated signatures for tolerance by global gene expression profiling of sequential samples of whole blood. The sequential measures reveal a 357-gene signature for immunoquiescence predicting as early as one year postoperatively the successful achievement of tolerance after drug withdrawal (p≤0.001).
Conclusion. These prospective longer term HLA-identical RT non-chimeric tolerance studies clarify, for the first time, tolerance predictive signatures based on both immunophenotypes for Tregs and serial monitoring of gene expression profiles.
To cite this abstract in AMA style:
Leventhal J, Mathew J, Salomon D, Kurian S, Friedewald J, Gallon L, Konieczna I, Tambur A, Levitsky J, Kanwar Y, Jie C, Abecassis M, Miller J. Non-Chimeric HLA-Identical Renal Transplant Tolerance: Regulatory Immunophenotypic/Genomic Biomarkers [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/non-chimeric-hla-identical-renal-transplant-tolerance-regulatory-immunophenotypicgenomic-biomarkers/. Accessed February 20, 2026.« Back to 2015 American Transplant Congress
