Early Conversion to Everolimus in De Novo Kidney Transplant Recipients: 12-Month Results from the ELEVATE Study
For the ELEVATE Study, Leiden, Netherlands.
Meeting: 2015 American Transplant Congress
Abstract number: 207
Keywords: Calcineurin, Efficacy, Proteinuria, Renal function
Session Information
Session Name: Concurrent Session: Kidney: Immunosupression Minimization
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:27pm-2:39pm
Presentation Time: 2:27pm-2:39pm
Location: Room 113-BC
Purpose: Long-term exposure to calcineurin inhibitors (CNIs) contributes to unfavorable long-term outcomes, including inferior renal function and premature graft loss with vascular lesions, glomerulosclerosis and tubule-interstitial fibrosis (IF/TA). Here, we present 12-month (M) data from the ELEVATE study (NCT01114529) designed to evaluate whether an early CNI to everolimus (EVR) conversion post-kidney transplant (KTx) better preserved renal function without compromising efficacy compared with standard CNI.
Methods: This 24-M, multicenter, open-label, controlled trial randomized (RND) de novo KTx recipients at 10–14 weeks post-Tx to convert from CNI to EVR (C0 6–10ng/mL) or remain on standard CNI therapy (C0, tacrolimus: 5–10ng/mL, cyclosporine: 100–250ng/mL). All patients received enteric-coated mycophenolate sodium (1080–1440mg/day) and steroids (≥5mg/day). The primary endpoint was change in estimated glomerular filtration rate (eGFR; MDRD4) from RND to M12. Composite endpoint of treated biopsy-proven acute rejection (BPAR; Banff ≥IB), graft loss, or death at M12 and safety were main secondary endpoints.
Results: A total of 717 patients were RND (EVR, N=360; CNI, N=357). Mean eGFR was significantly higher at all time-points after RND in EVR vs CNI group for intent-to-treat and on-treatment analyses (Figure). At M12, proteinuria (≥3g/day) was reported in 2/302 patients in EVR vs 1/326 patients in CNI group. Overall, 12.5% of patients on EVR returned to CNI therapy while 1.4% patients in CNI group switched CNI medication. At M12, the Kaplan-Meier incidence of composite efficacy failure rate was 5.9% in EVR vs 3.9% in CNI group (p=0.263). Overall incidence of BPAR was 9% in EVR vs 4.8% in CNI group. The incidence of graft loss (0.6% vs 0.8%) and death (1.5% vs 1.1%) was comparable. A similar proportion of patients in EVR and CNI groups reported adverse events (88.1% vs 82.7%) and serious adverse events (42.1% vs 39.8%).
Conclusion: Early conversion to EVR therapy at 10–14 weeks post-Tx vs continued CNI was associated with better preservation of renal function and comparable overall efficacy and safety.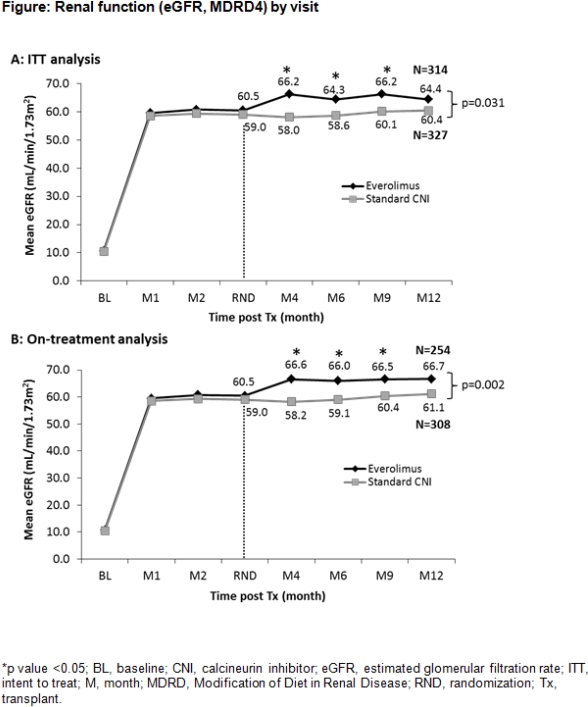
To cite this abstract in AMA style:
Fijter Jde, Holdaas H, Speziale A, Junge G, Wang Z, Cruzado J, Giet Mvander. Early Conversion to Everolimus in De Novo Kidney Transplant Recipients: 12-Month Results from the ELEVATE Study [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/early-conversion-to-everolimus-in-de-novo-kidney-transplant-recipients-12-month-results-from-the-elevate-study/. Accessed March 4, 2026.« Back to 2015 American Transplant Congress
