Perfusion Regulated Organ Therapeutics with Enhanced Controlled Testing (PROTECT): Novel Human Model to Mitigate Ischemia-Reperfusion Injury in Liver Transplant
Saint Louis University, Saint Louis, MO
Meeting: 2020 American Transplant Congress
Abstract number: C-349
Keywords: Ischemia, Preservation
Session Information
Session Name: Poster Session C: Ischemia Reperfusion & Organ Rehabilitation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Despite a global organ shortage, there is almost a 10% discard rate of ‘marginal donor livers’ (MDL), deemed unsuitable for transplantation due to the risk of ischemia-reperfusion (IR) injury. We hypothesized that targeting pathways regulating IR injury could enable successful transplants of such MDL. We developed a novel human split liver model to recapitulate such injury and enable therapeutic testing on each segment for rigorous control.
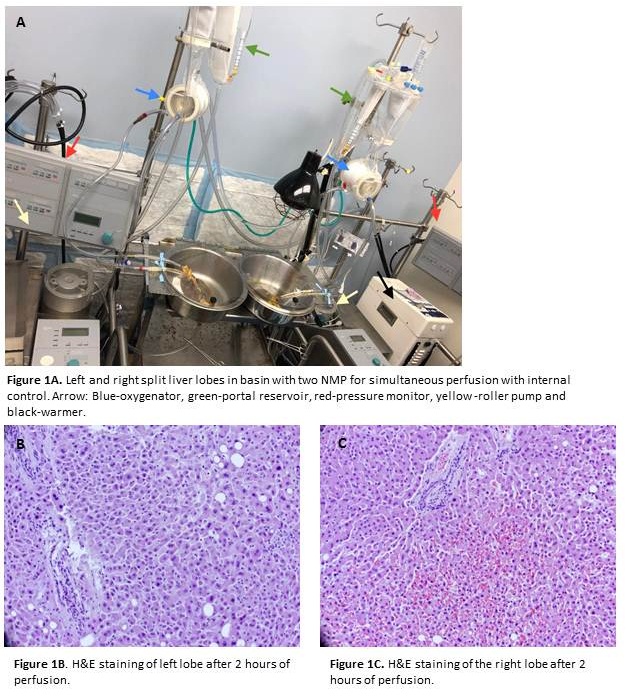
*Methods: Upon institutional approval, we procured human MDLs (n=5). An Extracorporeal Membrane Oxygenation machine was significantly modified to pump blood for hepatic and portal circulations (Figure 1A), designated as Perfusion Regulated Organ Therapeutics with Enhanced Controlled Testing (PROTECT) pump. Scheduled blood draws, liver biopsy, arterial blood gas, temperature and pressure readings were obtained. Two-tailed t-tests were conducted using a significance level of 0.05.
*Results: We successfully split and perfused MDL into a left lobe (LS) and right lobe (RS). In order to confirm that each lobe represented similar variability over time and thus a robust control, we measured a change in serology over the course of the 6-hour experiment for each lobe. Mean change (U/L) in ALT, AST was 1827, 1681 and 3369, 4464 for LS and RS respectively (p=0.92, p=0.54). We next evaluated surrogates for bile duct injury. While the mean change in serum bilirubin (mg/dL) was 0.2 for LS and 0.1 for RS (p=0.37), the mean change in GGT (U/L) was 24.66 for LS and 74 for RS (p=.27). Using a modified protocol for rigorous chemistry regulation we noted an improvement in synthetic surrogates attesting to the restoration of function. Liver histology was evaluated for hepatocellular necrosis, ductular changes, apoptosis as well as portal and lobular inflammation. No histological differences were noted between LS and RS. (Figure 1B-C) Successful temperature, pressure, and flow control were achieved in each segment. Based on these promising results we tested IR mitigators (n=2) using PROTECT, thus establishing proof of concept.
*Conclusions: Our PROTECT model represents a major development for controlled testing mitigating inter experimental variability noted in traditional systems. This model is a novel platform for interrogating pathways driving IR injury and in clinical applications for rapid translatability towards drug discovery and therapeutic testing to drive a paradigm change to organ recovery in transplant medicine.
To cite this abstract in AMA style:
Nazzal M, Madsen E, Nguyen M, Welu A, Madnawat H, Manithody C, Denton C, Blackall D, Carpenter D, Teckman J, Varma C, Jain A. Perfusion Regulated Organ Therapeutics with Enhanced Controlled Testing (PROTECT): Novel Human Model to Mitigate Ischemia-Reperfusion Injury in Liver Transplant [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/perfusion-regulated-organ-therapeutics-with-enhanced-controlled-testing-protect-novel-human-model-to-mitigate-ischemia-reperfusion-injury-in-liver-transplant/. Accessed March 4, 2026.« Back to 2020 American Transplant Congress