The State of Interventional Clinical Trials in Solid Organ Transplant: Insights from a Systematic Analysis of Clinicaltrials.gov
1Duke Clinical Research Institute, Durham, NC, 2Critical Path Institute, Tuscan, AZ, 3Fitzsimmons LLC, Chicago, IL, 4Duke University, Durham, NC
Meeting: 2020 American Transplant Congress
Abstract number: C-226
Keywords: Methodology, Multicenter studies, Rejection
Session Information
Session Name: Poster Session C: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The approach to immunosuppression after solid organ transplant has remained relatively unchanged for almost 20 years, despite the high prevalence of immunosuppression-related complications and suboptimal long-term graft survival. In order to better understand the limitations on new drug development for this patient population, we systematically reviewed all clinical trials conducted in the field of solid organ transplant between 2008 and 2016 using ClinicalTrials.gov, a comprehensive multinational database in which most clinical trials are prospectively registered.
*Methods: Using data publicly available on 9/3/2017, we identified all interventional clinical trials registered from January 2008 through December 2016 (n = 146,919) and applied disease condition terms provided by data submitters and medical subject heading terms generated by a National Library of Medicine algorithm to create a subset of only the solid organ transplant trials in the dataset (n = 589). We extracted key trial characteristics from registered protocol data and used descriptive statistics to summarize trial attributes, temporal trends, and distribution by type of organ transplant.
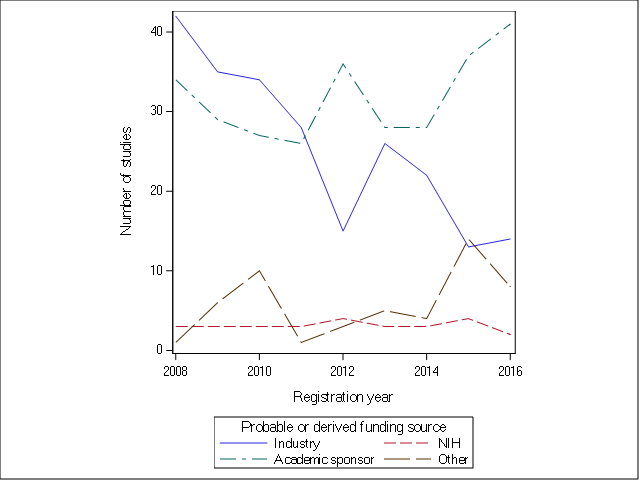
*Results: Studies of solid organ transplantation account for only 0.4% of all registered trials. The number of solid organ transplant trials registered each year trended down over time, from 80 in 2008 to 65 in 2016. The majority of clinical trials were small (median enrollment 54 [IQR 23, 120]), single center (65.0%), focused on kidney recipients (56.9%), and involved drug treatment (68.8%). Most trials (65.8%) were randomized, but of these only 29.9% used blinding. The most commonly evaluated primary outcomes were graft function (23.1%) and rejection (31.1%). The largest funding source (48.6%) was academic sponsors, and industry funding declined during the study period (Figure).
*Conclusions: There is very limited interventional clinical trial activity in the field of solid organ transplantation, presenting considerable barriers to the development and approval of new treatments. Creative approaches to better engage industry investment and promote expansion of NIH funded solid organ transplant networks are needed to improve the quality and number of studies in the field of solid organ transplantation. Ultimately, all key stakeholders will need to work together to improve long-term outcomes for solid organ transplant recipients.
To cite this abstract in AMA style:
Swaminathan AC, Li J, Chiswell K, McManigle W, O’Doherty I, Fitzsimmons W, Todd JL, Tibbs S, Palmer SM. The State of Interventional Clinical Trials in Solid Organ Transplant: Insights from a Systematic Analysis of Clinicaltrials.gov [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-state-of-interventional-clinical-trials-in-solid-organ-transplant-insights-from-a-systematic-analysis-of-clinicaltrials-gov/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress