Treatment of De Novo Renal Transplant Recipients with Calcineurin Inhibitor (CNI)-Free, Belatacept+Everolimus-Based Immunosuppression
1California Pacific Medical Center, San Francisco, CA, 2Colorado Kidney Care, Denver, CO, 3Hospital Marqués de Valdecilla, Cantabria, Spain, 4UVA Health, Charlottesville, VA, 5Saint Barnabas Medical Center, Livingston, NJ, 6Emory University School of Medicine, Atlanta, GA, 7UPMC Pinnacle, Harrisburg, PA, 8Bristol-Myers Squibb, Princeton, NJ, 9Yale University School of Medicine, New Haven, CT
Meeting: 2020 American Transplant Congress
Abstract number: B-104
Keywords: Calcineurin, Graft survival, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Compared with CNI-based immunosuppression, kidney transplant recipients receiving belatacept-based treatment present with a favorable metabolic profile, reduced chronic allograft nephropathy, and improved renal function, but with higher acute rejection (AR) rates during the peri-transplant period. We explored whether use of belatacept in combination with immunosuppressive agents other than those in the approved regimen would lead to AR rates comparable to those seen with CNI-based therapy.
*Methods: In this phase 2 study (NCT02137239), de novo renal transplant recipients were randomized (1:1) to receive belatacept + everolimus (BELA/EVL) or tacrolimus + mycophenolate mofetil (TAC/MMF) after thymoglobulin induction and a 7-day course of corticosteroids. Originally, 240 patients were slated for enrollment, but the study was prematurely discontinued (after 68 patients had been recruited) due to a belatacept supply constraint. The primary endpoint was the rate of biopsy-proven AR (BPAR) at Month 6.
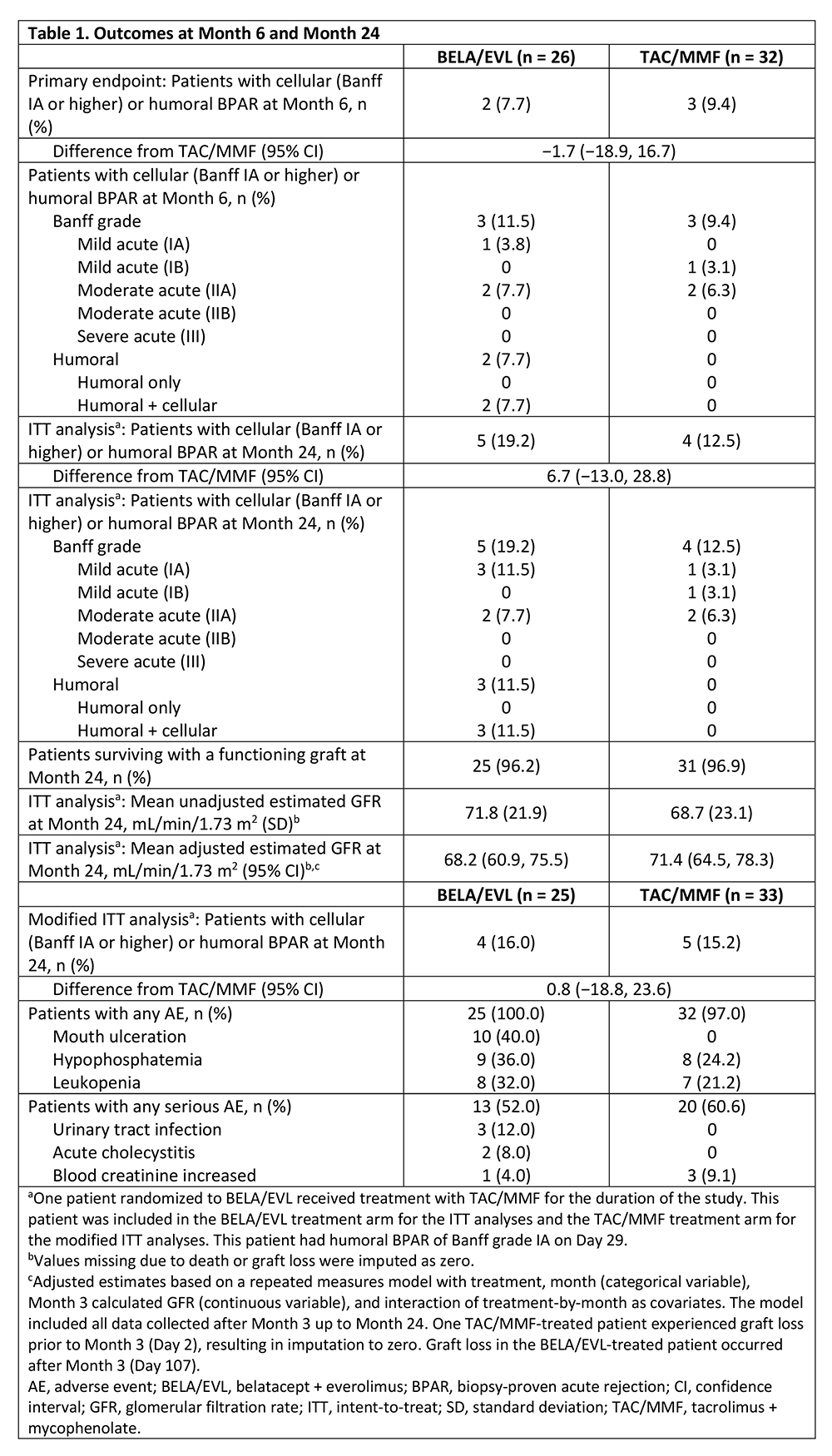
*Results: Outcomes are summarized in Table 1. Of the two BELA/EVL-treated patients with BPAR by Month 6, one had discontinued EVL 95 days prior to BPAR due to proteinuria, and the other began EVL on Day 6 post-transplant and continued until the time of BPAR on Day 15. Graft loss occurred in one BELA/EVL-treated patient (post-angioplasty for renal artery stenosis) and one TAC/MMF-treated patient (graft thrombosis due to a complicated vascular anastomosis). No deaths were reported during the study. Unadjusted, 4-variable, Modification of Diet in Renal Disease-estimated glomerular filtration rate (eGFR) was higher for BELA/EVL than for TAC/MMF, whereas the adjusted eGFR was lower for BELA/EVL than for TAC/MMF. Gastrointestinal post-transplant lymphoproliferative disorder (PTLD) without central nervous system (CNS) involvement developed in one BELA/EVL-treated patient. One TAC/MMF-treated patient developed CNS PTLD. No new or unexpected AEs were seen with either regimen.
*Conclusions: These data suggest that use of BELA with EVL may be associated with BPAR rates comparable to those seen with CNI-based treatment.
To cite this abstract in AMA style:
Peddi VR, Marder B, Gaite L, Oberholzer J, Goldberg R, Pearson T, Yang H, Allamassey L, Gao S, Polinsky M, Formica RN. Treatment of De Novo Renal Transplant Recipients with Calcineurin Inhibitor (CNI)-Free, Belatacept+Everolimus-Based Immunosuppression [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/treatment-of-de-novo-renal-transplant-recipients-with-calcineurin-inhibitor-cni-free-belatacepteverolimus-based-immunosuppression/. Accessed February 9, 2026.« Back to 2020 American Transplant Congress