Cell-Free DNA (cfDNA) is Released by Necroptosis and Promotes Kidney Ischemia Reperfusion Injury
Microbiology and Immunology, Western University, London, ON, Canada
Meeting: 2020 American Transplant Congress
Abstract number: A-339
Keywords: Apoptosis, Inflammation, Mice, knockout, Natural killer cells
Session Information
Session Name: Poster Session A: Biomarker Discovery and Immune Modulation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Transplantation is invariably associated with ischemia reperfusion injury (IRI) which causes organ dysfunction. IRI is also directly linked to several forms of programmed cell death including apoptosis and necroptosis, which increase kidney dysfunction, promote inflammation and may contribute to premature graft failure. The contribution of necroptosis following kidney IRI to cell-free DNA (cfDNA) generation and the potential of cfDNA to activate effectors such as NK cells involved in kidney IRI have not been defined.
*Methods: cfDNA release was tested in vitrousing mouse B6 microvascular endothelial cells (MVEC), following exposure to TNFα, Smac-mimetic (inhibitor of Inhibitor of Apoptosis Proteins), and IETD (caspase-8 inhibitor) to induce programmed cell death. cfDNA was isolated from supernatant using a NucleoSpin PCR Clean-up protocol, and quantified using Quant-iT PicoGreen dsDNA Assay Kits and 480/520 nm fluorometry. In vivo, IRI was induced by renal clamping in uni-nephrectomized mice. NK cells were purified from spleens of WT B6 mice and exposed to cfDNA isolated from necroptotic cells. qPCR was used to quantify TNFα, IFNγ, and Granzyme-B as markers of activation.
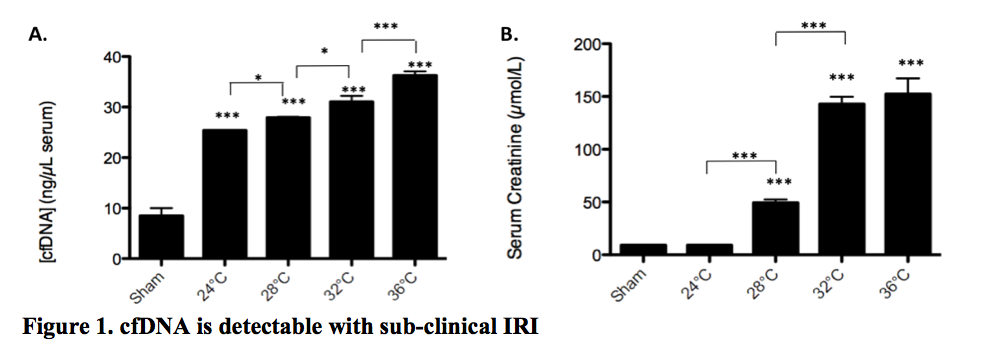
*Results: Necroptotic (TNFα+Smac +IETD) MVEC released considerably more cfDNA than apoptotic MVEC (TNFα +Smac) or untreated controls (n=8, p<0.0001). cfDNA was readily detected in serum following kidney IRI in vivo. Levels in genetically altered mice incapable of both apoptosis and necroptosis (RIPK3-/-/caspase-8-/-, DKO) were comparable to sham IRI controls (n=7, p<0.0001). cfDNA appears to be a sensitive biomarker of injury and serum levels were reduced even with small reductions in IRI model temperatures (Figure 1). Furthermore cfDNA was detectable in subclinical IRI at 24-28°C, in which serum creatinine remained in the normal range (n=4, p<0.0001 vs control). NK cells are well described IRI effectors. NK cells treated with 100 ng/mL cfDNA were activated with upregulation of Granzyme B (p<0.0001), IFNγ (p<0.0001), and TNFα (p<0.01).
*Conclusions: cfDNA is generated with programmed cell death and particularly with necroptosis in endothelial cells. Serum cfDNA is a sensitive biomarker of injury and is detectable even in subclinical IRI. cfDNA, by its direct ability to activate NK cells, can promote IRI-associated inflammation and trigger further cell death with cfDNA release. Strategies that reduce inflammatory injury and necroptosis that release cfDNA, would be expected to attenuate IRI and improve kidney function following transplantation.
To cite this abstract in AMA style:
Dionne AC. Cell-Free DNA (cfDNA) is Released by Necroptosis and Promotes Kidney Ischemia Reperfusion Injury [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/cell-free-dna-cfdna-is-released-by-necroptosis-and-promotes-kidney-ischemia-reperfusion-injury/. Accessed February 20, 2026.« Back to 2020 American Transplant Congress