Impact of Eplet Mismatches on the Risk of Graft Loss: Not All Eplets are Created Equal
1Medicine, McGill University, Montreal, QC, Canada, 2Organ Donation and Transplantation, Canadian Blood Services, Ottawa, ON, Canada, 3Emory University Hospital, Atlanta, GA, 4Montreal Children's Hospital, Montreal, QC, Canada, 5Medicine, University of British Columbia, Vancouver, BC, Canada, 6Université du Québec À Montréal, Montreal, QC, Canada
Meeting: 2020 American Transplant Congress
Abstract number: 620
Keywords: Epitopes, Graft failure, Immunogenicity, Kidney transplantation
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes VI
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
*Purpose: To mitigate risks related to HLA incompatibility, we assessed whether some unique sequence defined HLA targets (eplets) are associated with graft failure while others are not. We further assessed whether higher risk eplets are differentially distributed within donor and candidate pools.
*Methods: To assess risk of death-censored graft failure associated with individual eplet mismatches, we fit multivariable Cox proportional hazards models in a cohort of 118 382 unsensitized (PRA 0%) US first kidney transplant recipients (2000 to 2015) from the scientific registry of transplant recipients. Allele-level HLA genotypes (A, B, C, DRB1 and DQB1) were imputed using an algorithm from the National Marrow Donor Program. HLA genotypes were translated to epitypes with 449 potential eplets (223 Class I: 72 antibody-verified (AbVer), 151 non-antibody-verified (non-AbVer), 226 Class II: 72 AbVer, 154 Non-AbVer) considered. Models were adjusted for recipient, donor and transplant characteristics. Hazard ratio (HR) estimates with false discovery corrected p-value threshold < 0.0001 were deemed statistically significant. Eplet distributions in donor (N=241 738) and candidate (N= 140 911) pools were also measured.
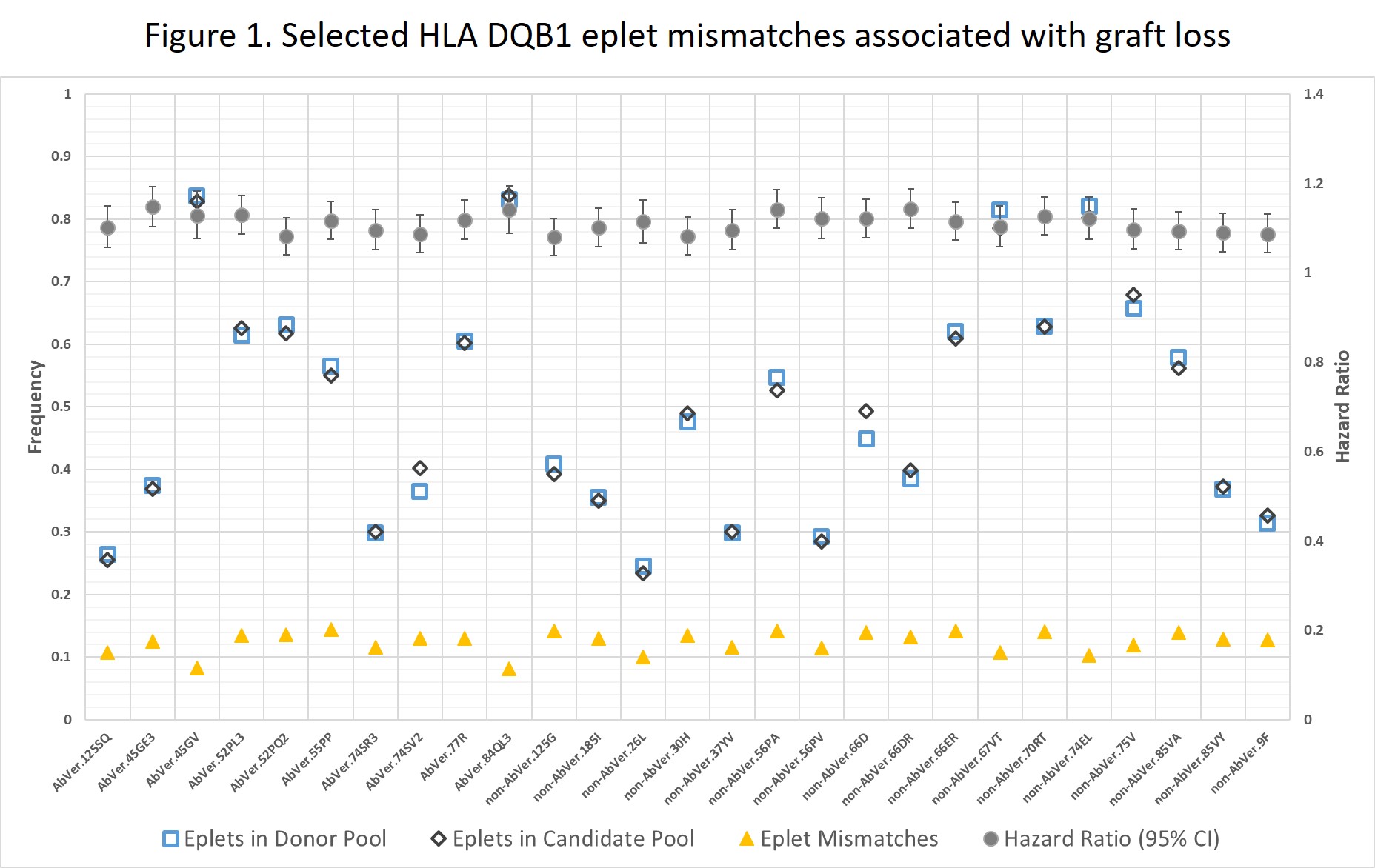
*Results: Of 449 eplets, a total of 410 eplet mismatches were observed; of those 154 (75 class I: 30 AbVer and 45 non-AbVer, and 79 class II: 33 AbVer and 46 non-AbVer) were statistically significantly associated with an increased risk of death-censored graft failure. Eplet distributions in donor and candidate pools did not differentially inform eplet mismatches associated with death-censored graft failure versus not. Figure 1 presents selected HLA-DQB1 eplet mismatches associated with graft loss and their distributions in donor and recipient pools.
*Conclusions: Our analysis identified a subset of AbVer and non-AbVer HLA class I and II eplet mismatches associated with increased risk of graft loss. These findings set the stage for understanding determinants of immune dominant mismatches. A strategy prioritizing higher risk eplets could enhance feasibility and improve utility of donor:recipient eplet matching.
To cite this abstract in AMA style:
Mohammadhassanzadeh H, Klement W, Gebel H, Foster B, Keown P, Oualkacha K, Sapir-Pichhadze R. Impact of Eplet Mismatches on the Risk of Graft Loss: Not All Eplets are Created Equal [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-eplet-mismatches-on-the-risk-of-graft-loss-not-all-eplets-are-created-equal/. Accessed March 5, 2026.« Back to 2020 American Transplant Congress