Comparing Weight-Based Dosing of Envarsus XR in Obese and Non-Obese Renal Transplant Recipients
North Shore University Hospital, Manhasset, NY
Meeting: 2020 American Transplant Congress
Abstract number: 608
Keywords: Calcineurin, Dosage, Kidney transplantation, Obesity
Session Information
Session Name: All Organs: Pharmacogenomics / Pharmacokinetics
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: Tacrolimus has a narrow therapeutic window and significant inter- and intra-patient variability leading to variations in drug concentration. The recommended initial weight-based dose of extended-release tacrolimus (LCPT; Envarsus XR) in kidney transplant recipients is 0.14 mg/kg/day. However, no data exist regarding weight-based LCPT dosing for obese patients. With significant differences between ideal body weight (IBW), adjusted body weight (ABW) and total body weight (TBW) in obese patients, doses may vary greatly based on the weight chosen, thereby affecting therapeutic concentrations.
*Methods: Single-center retrospective chart review of kidney transplant recipients initiated on de novo LCPT from July 1 to November 1, 2019. Multi-organ recipients, re-transplants on tacrolimus and patients with a gastric sleeve were excluded. The primary outcome was weight-based dosing requirements (mg/kg/day) to reach a therapeutic concentration (8-12 ng/mL) for obese kidney transplant recipients compared to non-obese recipients. Secondary outcomes include time to therapeutic concentrations and characterizing the optimal weight-based approach for dosing LCPT.
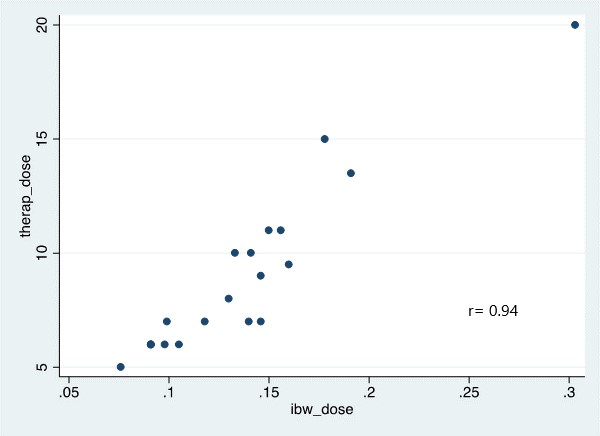
*Results: Of the 31 kidney transplant recipients, 13 (42%) were obese. Baseline characteristics were similar between both cohorts. The mean therapeutic dose was 0.13 mg/kg vs. 0.14 mg/kg in the obese and control groups based on IBW, respectively, (p=0.839). This finding was supported by a strong linear relationship between IBW and therapeutic dose (r = 0.94, p<0.001) vs. r=0.84 for TBW (Figure 1). In a multivariate analysis, age, sex, race, BMI, induction agent and delayed graft function did not influence therapeutic dose. Mean time to therapeutic concentration was 4 ± 1.75 days in both cohorts.
*Conclusions: In both obese and non-obese kidney transplant recipients, IBW had a stronger correlation with the therapeutic dose for LCPT. In addition, as BMI increased the therapeutic dose did not change, suggesting that IBW is the optimal weight to utilize in this population. Further pharmacokinetic studies are warranted to support these findings.
To cite this abstract in AMA style:
Breslin N, Jandovitz N, Nair V, Abate M, Teperman L. Comparing Weight-Based Dosing of Envarsus XR in Obese and Non-Obese Renal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/comparing-weight-based-dosing-of-envarsus-xr-in-obese-and-non-obese-renal-transplant-recipients/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress