Impact of CYP3A5 Phenotype on Early Time in Therapeutic Range among Pediatric Renal and Heart Transplant Recipients
University of Michigan, Ann Arbor, MI
Meeting: 2020 American Transplant Congress
Abstract number: 604
Keywords: Calcineurin, Gene polymorphism, Pediatric
Session Information
Session Name: All Organs: Pharmacogenomics / Pharmacokinetics
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: Tacrolimus time in therapeutic range (TTR) as a measure of intra-patient variability has been associated with poor long-term outcomes. Multiple factors may contribute to TTR but few have been evaluated. The purpose of this study was to investigate the impact of CYP3A5 phenotype on TTR early post-transplant in pediatric patients.
*Methods: This was a retrospective analysis of all pediatric patients who underwent renal or heart transplant from 6/1/14 to 12/31/18 and were initiated on tacrolimus with whole blood or buffy coat samples available for secondary use. Samples were genotyped for CYP3A5*3, CYP3A5*6, and CYP3A5*7 and categorized as CYP3A5 expressers (CYP3A5*1) or CYP3A5 non-expressers. TTR was calculated for the first 90 days post-transplant using the Rosendaal linear interpolation method. Target tacrolimus levels were 8-12ng/mL in kidney recipients and 10-15 ng/mL in heart recipients. Per protocol, initial dose of tacrolimus was 0.1 mg/kg twice daily. The primary outcome compared 90-day TTR between CYP3A5 expressers and non-expressers using simple and multiple linear regression.
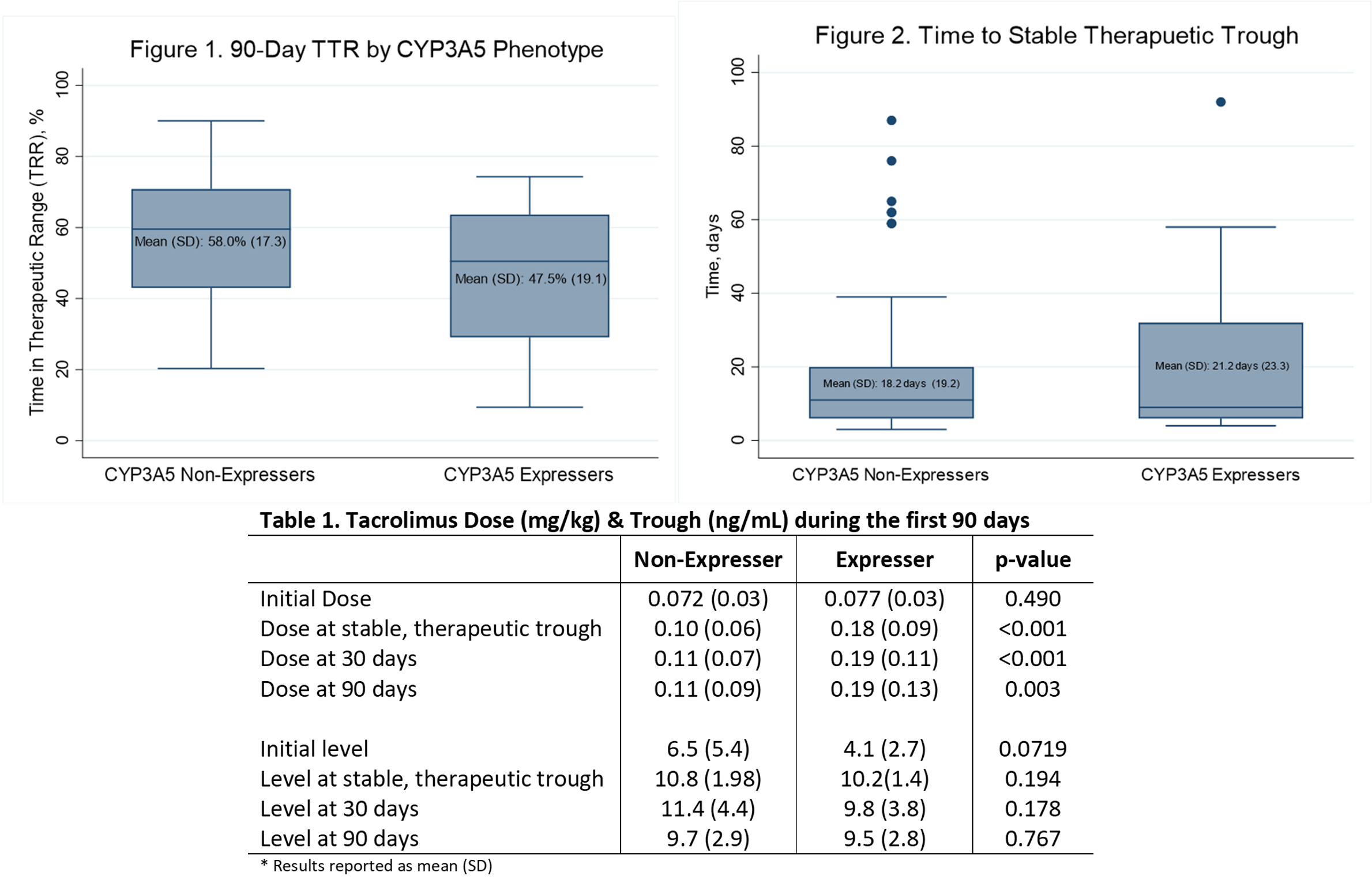
*Results: A total of 85 pediatric transplant patients were analyzed; 37 heart recipients and 48 kidney recipients. Nineteen patients were CYP3A5 expressers and 66 were CYP3A5 non-expressers. TTR in the first 90 days post-transplant was significantly higher among non-expressers (57.95% vs 47.29%, p=0.026; figure 1). The difference remained significant when controlling for organ type and initial dose (p= 0.029).As expected, CYP3A5 expressers required a higher dose at all evaluated timepoints (table 1). However, there was no difference in the time to the first stable, therapeutic trough defined as ≥2 sequential troughs within target (18.2 days vs 21.2 days, p=0.057; figure 2). There was also no difference when controlling for organ type and initial dose (p=0.346). A post hoc analysis of 30-day TTR also found no difference by CYP3A5 phenotype (49.1% vs 46.75%, p=0.67).
*Conclusions: CYP3A5 phenotype is associated with TTR early post-transplant beyond the known increase in time to first therapeutic level. Future work should further explore the benefit of genotype directed dosing on TTR.
To cite this abstract in AMA style:
Leino A, Pasternak A. Impact of CYP3A5 Phenotype on Early Time in Therapeutic Range among Pediatric Renal and Heart Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-cyp3a5-phenotype-on-early-time-in-therapeutic-range-among-pediatric-renal-and-heart-transplant-recipients/. Accessed February 9, 2026.« Back to 2020 American Transplant Congress