Induction Immunosuppression in Primary Pediatric Liver Transplant Recipients
1Surgery, Division of Transplantation, University of Minnesota, Minneapolis, MN, 2Division of Renal Diseases and Hypertension, University of Minnesota, Minneapolis, MN, 3Biostatistics, University of Minnesota, Minneapolis, MN
Meeting: 2020 American Transplant Congress
Abstract number: 585
Keywords: Graft survival, Immunosuppression, Induction therapy, Pediatric
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
*Purpose: Induction immunosuppression for pediatric liver transplants varies widely among centers. We sought to evaluate the impact of different induction regimens on patient and graft survival.
*Methods: SRTR database was used to identify all pediatric primary liver transplant recipients between 2002-2016 who were discharged on tacrolimus, or tacrolimus and mycophenolate maintenance with or without steroids. Patients were grouped by type of induction into no induction, depletional, and non-depletional (IL-2) groups. Mixed effects proportional hazards and logistic regression models were fit to assess the association of induction therapy on graft and patient survival, and acute rejection in the first 6 and 12 months after adjusting for recipient and donor characteristics. Transplant center was included as a random effect in these models.
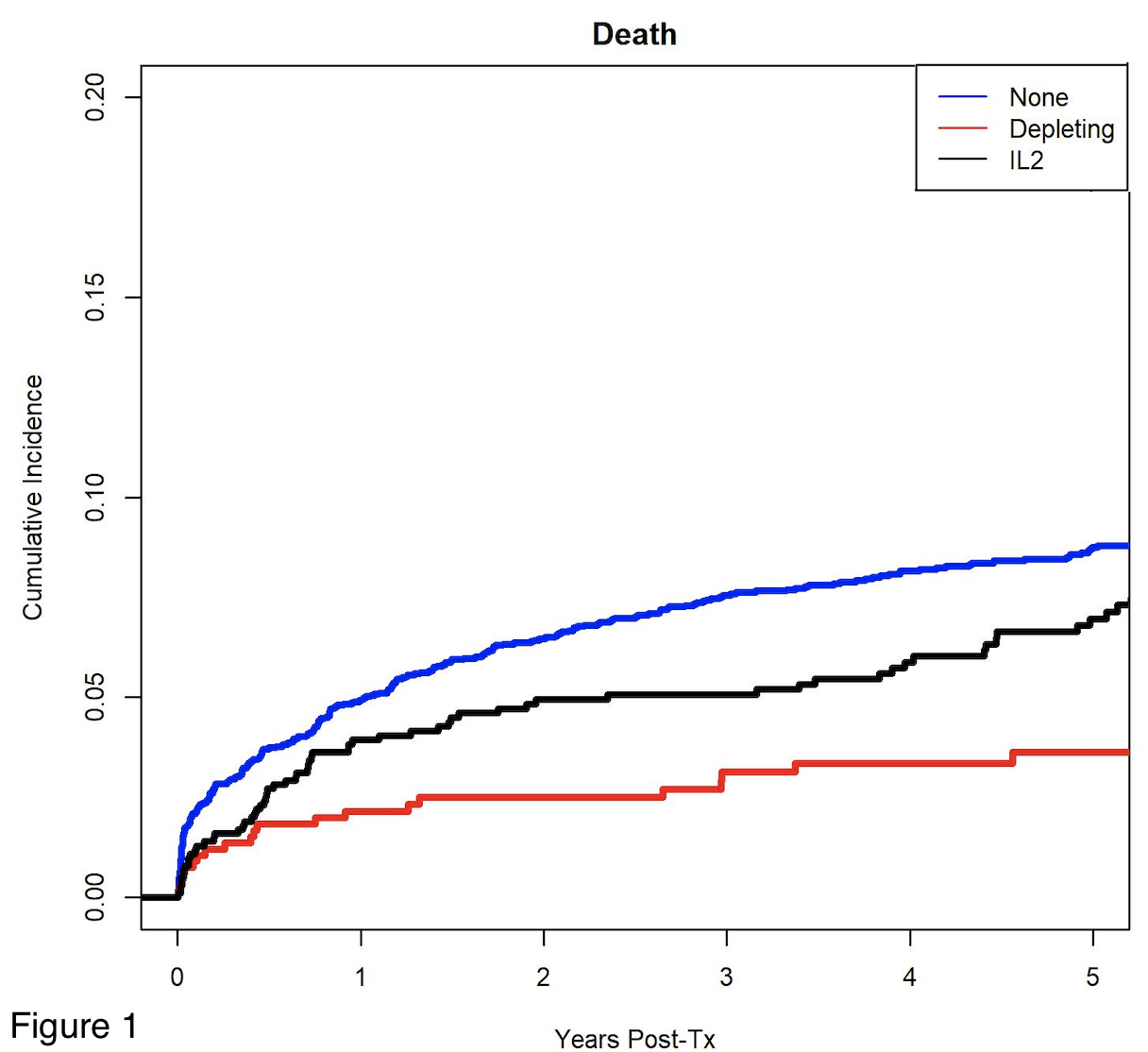
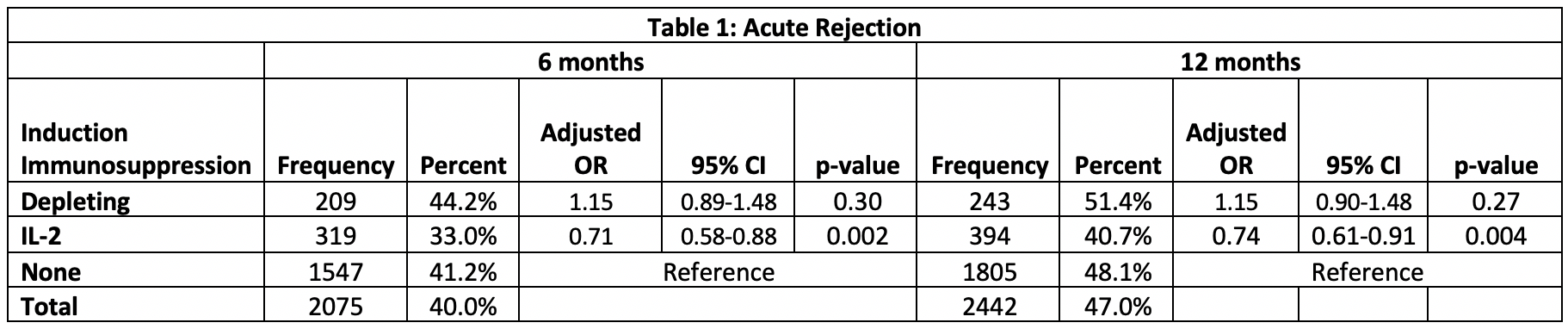
*Results: 5194 recipients met inclusion criteria, 473 received depletional induction, 968 received IL-2 inhibitors and 3753 received no induction. Patients receiving depletional (aHR:0.59, 95% CI:0.39-0.91) and IL-2 (aHR:0.88, 95% CI:0.45-1.72) had higher patient survival compared to those receiving no induction therapy (Figure 1). There were no significant differences in graft survival between the groups (p=0.13). Those receiving IL-2 were less likely to have an acute rejection episode within the first 12 months post-transplant compared to those receiving no induction (aOR: 0.74, 95% CI: 0.61-0.91, p=0.004), but there was no difference in acute rejection between those receiving depletional and no induction (aOR:1.15, 95% CI:0.89-1.48) (Table 1). There was no difference in the cause-specific hazard of death due to infection (p=0.63) among the groups.
*Conclusions: In this large cohort of pediatric primary liver transplant recipients, use of depletional induction was associated with higher patient survival. Use of IL-2 inhibitors was associated with lower rejection rates within 12 months. Our findings suggest that a biologic induction may be beneficial in primary pediatric liver transplant recipients.
To cite this abstract in AMA style:
Shaker T, Ramanathan K, Riad S, Vock D, Chinnakotla S. Induction Immunosuppression in Primary Pediatric Liver Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/induction-immunosuppression-in-primary-pediatric-liver-transplant-recipients/. Accessed January 4, 2026.« Back to 2020 American Transplant Congress