Anti-HLA IgM Antibodies are Unaffected by Imlifidase (IdeS) Treatment
1Hansa Biopharma AB, Lund, Sweden, 2Section of Transplantation Surgery, Department of Surgical Sciences, Uppsala, Sweden
Meeting: 2020 American Transplant Congress
Abstract number: 572
Keywords: Highly-sensitized, HLA antibodies, Induction therapy, Kidney transplantation
Session Information
Session Name: Kidney Immunosuppression: Desensitization
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
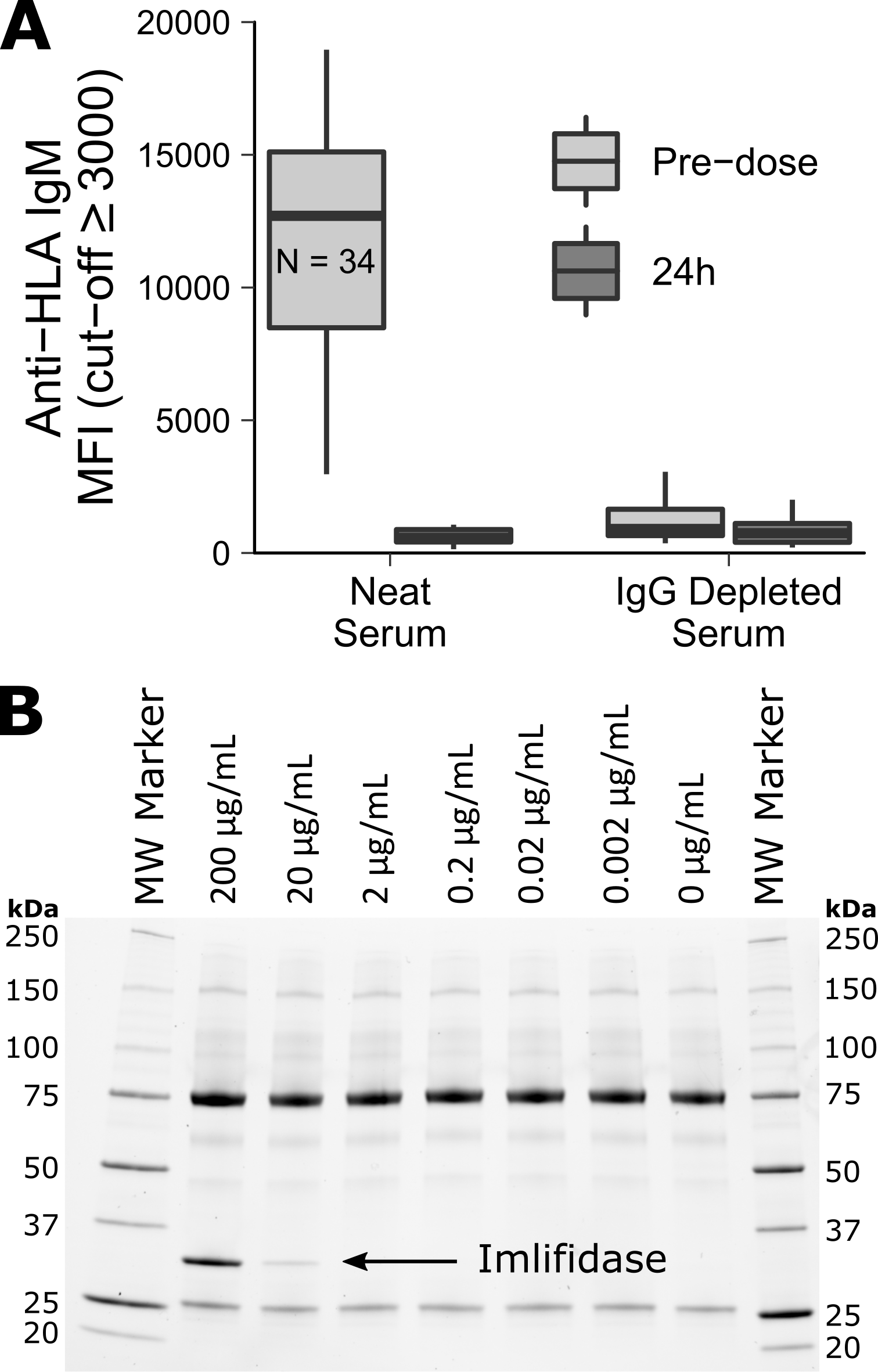
*Purpose: The immunoglobulin G (IgG)-degrading cysteine protease, imlifidase (IdeS) is an IgG endopeptidase under development as a rapid desensitization treatment in kidney transplantation. Imlifidase is highly specific and cleaves all subclasses of human IgG. Imlifidase inactivates IgG-dependent Fc-dependent effector functions, inhibiting antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis (ADCP) and complement deposition (CDC) within hours after a single dose in clinical trials. The study purpose was to further assess whether imlifidase has an effect on human anti-HLA IgM antibodies (Abs) in sensitized patients with end stage renal disease (ESRD).
*Methods: Serum samples from sensitized patients with ESRD enrolled in a phase II trial (13-HMedIdeS-02; NCT02224820) and treated with imlifidase at 0.12 or 0.25 mg/kg were investigated. Samples were collected pre-dose and 24h post imlifidase administration to measure the level of anti-HLA IgM Abs. Patient sera were total IgG-depleted using CaptureSelect. In addition, pre-dose samples and purified human IgM samples were treated with imlifidase in vitro and evaluated by SDS-PAGE gel, Western blot and LABScreen Single Antigen HLA (IgG, IgM) (One Lambda). Samples were DTT-treated before separation on SDS-PAGE gels and blotted and probed with PE-anti-human IgM (One Lambda). Purified human IgM was incubated with different concentrations of imlifidase to assess possible cleavage of IgM.
*Results: Neat and IgG-depleted sera from patients with ESDR were analyzed using LABScreen HLA-IgG and HLA-IgM detectors. A reduction of anti-HLA IgG Abs was observed 24h post imlifidase treatment. A similar reduction was seen in two out of seven patients using anti-HLA IgM detection reagent (Fig 1A). After removal of IgG by CaptureSelect the remaining anti-HLA IgM mean fluorescence intensity (MFI) signals were not reduced by imlifidase treatment (Fig 1A). No digestion of purified human IgM was seen after incubation with imlifidase (Fig 1B). Cleavage of IgM from patients with ESRD by imlifidase was not measurable using a PE-anti-human IgM detection reagent in Western blot analysis (data not shown).
*Conclusions: We conclude that human IgM is not cleaved by imlifidase. The reduction of anti-HLA IgM signals, which was noted after imlifidase treatment in some patients, was not observed in IgG-depleted sera. The high anti-HLA IgM signals are likely to be an artefact of IgG-complexed IgM on the surface of HLA beads.
Figure 1.
To cite this abstract in AMA style:
Runström A, Bockermann R, Sjöholm K, Roupé KM, Schiött Å, Winstedt L, Kjellman C, Lorant T. Anti-HLA IgM Antibodies are Unaffected by Imlifidase (IdeS) Treatment [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/anti-hla-igm-antibodies-are-unaffected-by-imlifidase-ides-treatment/. Accessed February 17, 2026.« Back to 2020 American Transplant Congress