Association of Kidney Allograft Fibrosis-Associated Proteins with Chronic Lung Allograft Dysfunction
1Nephrology, Toronto General Hospital Research Institute and Multi-Organ Transplant, University Health Network, Toronto, ON, Canada, 2Lung Transplantation, Toronto Lung Transplant Program, University Health Network, Toronto, ON, Canada
Meeting: 2020 American Transplant Congress
Abstract number: 530
Keywords: Graft failure, Lung transplantation, Peptides
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes V
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
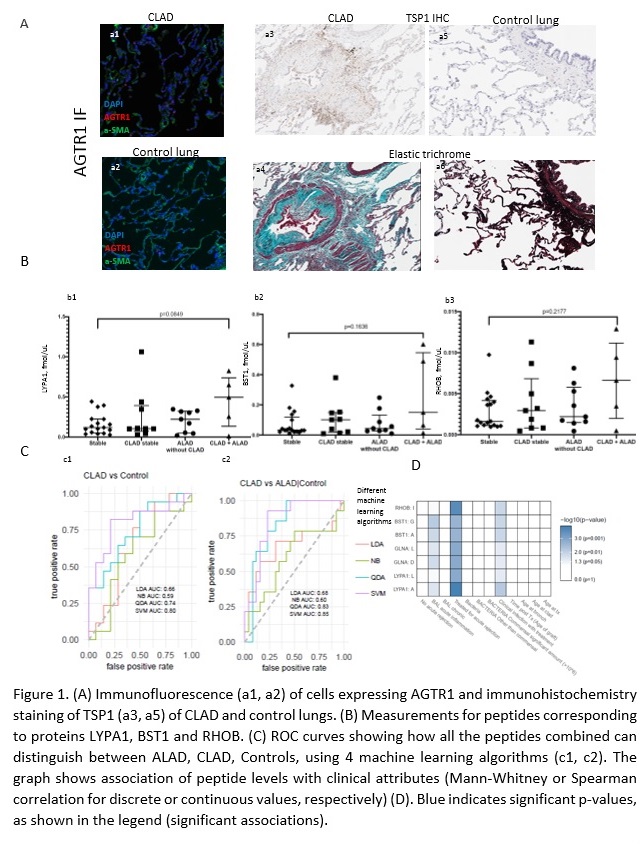
*Purpose: Chronic lung allograft dysfunction (CLAD) remains the leading cause of long-term mortality after lung transplant. Non invasive biomarkers that could assist in early diagnosis and patient management are lacking. Renin-angiotensin-aldosterone system (RAAs), is involved in fibrogenesis in both kidney and lung disease. We previously identified 6 RAAs-regulated proteins (RHOB, BST1, LYPA1, GLNA, TSP1, LAMB1) in kidney cells, that may play a role in fibrosis in vivo and demonstrated that their urine excretion rate correlates with allograft fibrosis in kidney transplant recipients. We hypothesize that the RAAs system is activated in CLAD lungs and that the same RAAs-regulated proteins could be used as biomarkers of CLAD in Bronchoalveolar Lavage (BAL).
*Methods: We performed immunofluorescence staining of angiotensin receptors (AGTR1 and AGTR2) and immunohistochemical staining of TSP1 in 10 explanted CLAD lungs, compared to 5 control lungs. We developed mass spectrometry-based assays targeting two peptides for each of the 6 RAAs-regulated proteins and we monitored them in our training set of BAL samples from 40 lung transplant recipients.
*Results: We observed significantly more AGTR1+ cells in CLAD versus control lungs (p=0.04) (Fig 1A, a1 and a2) and a trend towards higher expression of TSP1 (Fig 1A, a3 -a6) in CLAD samples compared to controls. MS analysis showed that RHOB, BST1 and LYPA1 peptides were numerically higher in the BAL of CLAD patients with concomitant acute >10% decline in lung allograft function (CLAD-ALAD) in comparison to patients with stable lung function (Fig 1B). Importantly, all peptides combined had a predictive value for CLAD of >0.8 when using QDA and SVM machine learning algorithms (Fig 1C), and correlated most significantly with the degree of acute inflammation and rejection in lung allografts (Fig 1D).
*Conclusions: Our pilot data support the hypothesis that RAAs is involved in CLAD and that RAAs-regulated proteins may help identify patients with active CLAD. We are currently validating these findings using a larger cohort and established endpoints.
To cite this abstract in AMA style:
Farkona S, Berra G, Mohammed-Ali Z, Ly P, Levy L, Renaud-Picard B, Zehong G, Daigneault T, Juvet S, McEvoy C, Clotet-Freixas S, Martinu T, Konvalinka A. Association of Kidney Allograft Fibrosis-Associated Proteins with Chronic Lung Allograft Dysfunction [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/association-of-kidney-allograft-fibrosis-associated-proteins-with-chronic-lung-allograft-dysfunction/. Accessed February 16, 2026.« Back to 2020 American Transplant Congress