Role of CAR-T Cell Therapy in Post Transplant Lymphoproliferative Disorder
S. Krishnamoorthy, A. Malone, R. Delos Santos, H. Murad, T. Alhamad
Transplant, Washington University in St. Louis, Saint Louis, MO
Meeting: 2020 American Transplant Congress
Abstract number: 504
Keywords: Epstein-Barr virus (EBV), Post-transplant lymphoproliferative disorder (PTLD), T cells
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:27pm-4:39pm
Presentation Time: 4:27pm-4:39pm
Location: Virtual
*Purpose: Chimeric Antigen Receptor (CAR) T cells are a genetically modified autologous immunotherapy that uses a patient’s own T cells modified with a chimeric antigen receptor that directs it against lymphoma cells. Though CAR-T cells have shown activity against diffuse large B cell lymphomas (DLBCL) in patients who have second or later relapses or are chemo-resistant to salvage therapy, its use and outcomes in solid organ recipients with post transplant lymphoproliferative disorder (PTLD) have not been defined. Using this case series, we review our experience with CAR-T cell therapy in solid organ transplant recipients at our center.
*Methods: Retrospective review of electronic medical records of solid organ transplant recipients who received CAR-T therapy between January 2018 and November 2019.
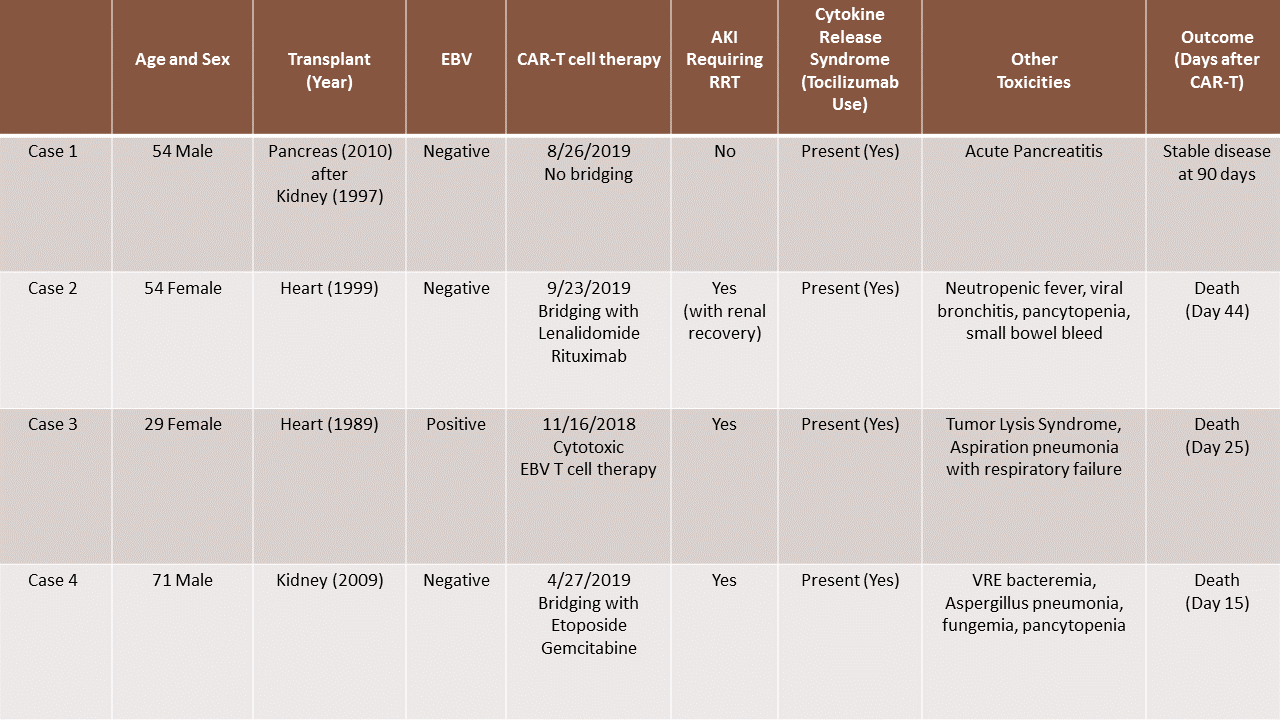
*Results: 4 cases identified. 2 cases of orthotopic heart transplant (OHT), 1 case of deceased donor kidney transplant and 1 case of pancreas after kidney transplant (PAK). These patients developed PTLD about 10-20 years after their transplant. All patients showed progression of disease without remission after first and second line chemotherapy. Only 1 of the patients had EBV positive PTLD and was treated with EBV cytotoxic T cell therapy, while the other 3 underwent pheresis and proceeded to get CAR-T cell therapy. All patients developed cytokine release syndrome, neurotoxicity and received tocilizumab. 3 out of 4 patients developed AKI requiring renal replacement therapy (RRT). Only 1 of these 3 recovered renal function. All 3 patients who required RRT either transitioned to hospice or deescalated care and did not survive. The PAK patient who developed acute pancreatitis post immunotherapy without kidney injury has shown continued response to therapy without new lymphomatous involvement 90 days after CAR-T therapy.
*Conclusions: This series identifies the challenges of managing PTLD and immunosuppression in solid organ recipients with CAR-T therapy given poor outcomes and significant toxicity in this subgroup.
To cite this abstract in AMA style:
Krishnamoorthy S, Malone A, Santos RDelos, Murad H, Alhamad T. Role of CAR-T Cell Therapy in Post Transplant Lymphoproliferative Disorder [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/role-of-car-t-cell-therapy-in-post-transplant-lymphoproliferative-disorder/. Accessed January 20, 2026.« Back to 2020 American Transplant Congress