The Urine Qisant Score: Non-Invasive, Accurate, Diagnosis and Prediction of Kidney Transplant Rejection
1University of California San Francisco, San Francisco, CA, 2University of Mexico, Instituto Nacional De Ciencias Medicas y Nutricion, San Francisco, CA
Meeting: 2020 American Transplant Congress
Abstract number: 457
Keywords: Area-under-curve (AUC), Kidney transplantation, Non-invasive diagnosis, Rejection
Session Information
Session Name: Kidney: Acute Cellular Rejection
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
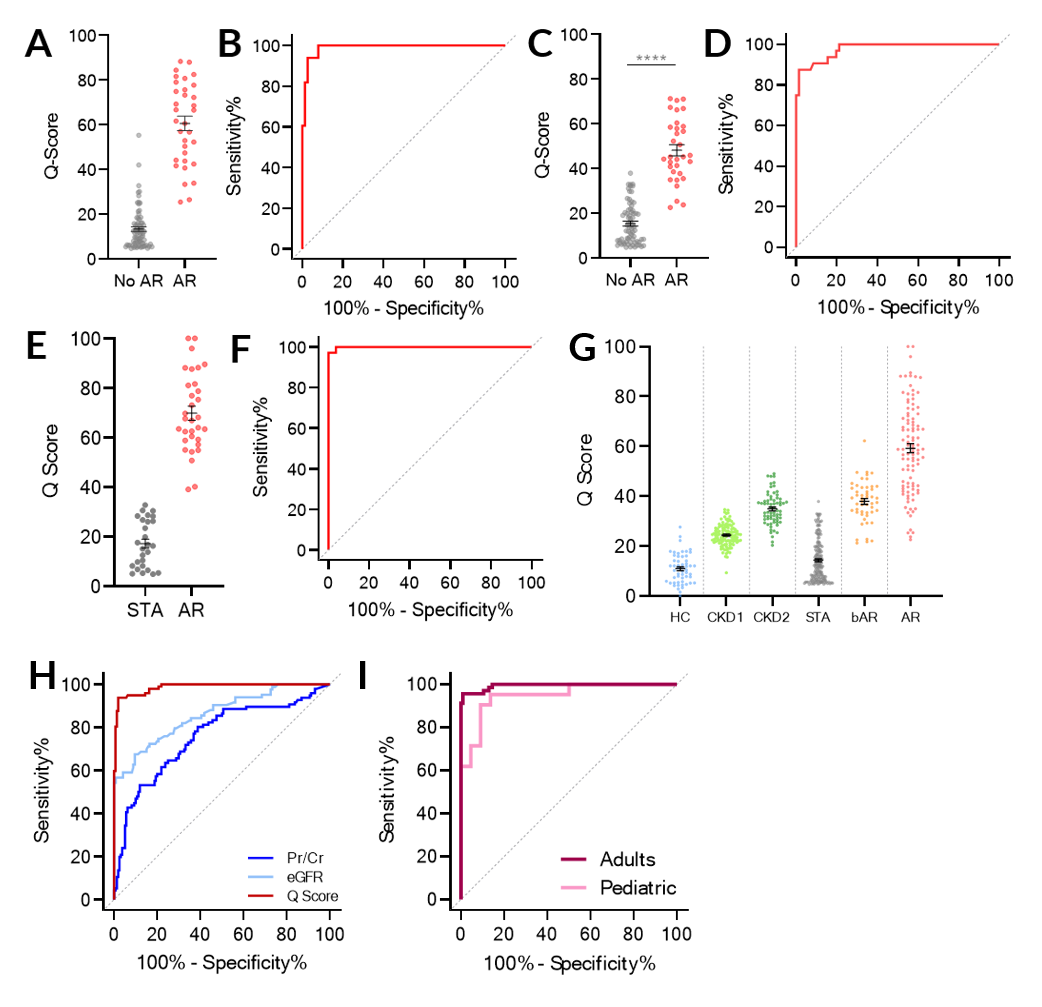
*Purpose: Accurate and non-invasive monitoring of renal allografts post-transplant is essential for early detection of acute rejection (AR) and to directly impact the long-term survival of the transplant. We present the development and validation of a noninvasive, spot urine-based diagnostic assay, QiSant, based on measurements of six urinary DNA, protein and metabolic biomarkers.
*Methods: In this multicenter, prospective study, a total of 601 patient samples were assessed. 365 urine samples belonged to renal transplant recipients, where every urine samples were paired with a renal transplant biopsy for phenotype classification into either of the diagnoses: stable (STA; n=170), acute rejection (AR; n=103), borderline AR (bAR; n=65) and BK virus nephropathy (BKVN; n=9). An additional 32 patients with AR had urine samples collected prior to the rejection episode; these samples were also paired with biopsies.cfDNA, m-cfDNA, clusterin, creatinine, total protein, and CXCL10 were measured in the urine supernatant collected from patients.
*Results: Through a comparison to contemporaneous renal transplant biopsy results, we demonstrate that a urinary composite QiSant-score (Q-Score) enables diagnosis of AR after renal transplantation, with an area under the receiver operating characteristic curve of 0.987 across the training and two validation sets (Figures A – F). The Q-Score was highest in AR patients, lowest in stable patients, and intermediate with borderline-AR patients (Figure G). In addition, we also highlight the clinical utility of this assay for predicting AR over that of Pr/Cr and eGFR, enabling earlier detection of rejection than currently possible by standard of care tests (Figure H). Lastly, we demonstrate that the Q-Score performed equally well in adult and pediatric patients.
*Conclusions: In conclusion, the Q-Score offers the potential to be used as an immune monitoring tool to guide the use of immunosuppression, with the ultimate goal of controlling subclinical intragraft inflammation and thus prolonging graft survival. Currently additional studies are underway to evaluate the potential of the assay to substitute protocol renal transplant biopsies for the determination of clinical and subclinical rejection, to allow for early detection and proactive management of graft rejection, and thus improve both patient morbidity and graft survival.
To cite this abstract in AMA style:
Yang JY, Sarwal R, Sigdel T, Damm I, Rosenbaum B, Liberto J, Chan-On C, Alberu J, Vincenti F, Sarwal MM. The Urine Qisant Score: Non-Invasive, Accurate, Diagnosis and Prediction of Kidney Transplant Rejection [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-urine-qisant-score-non-invasive-accurate-diagnosis-and-prediction-of-kidney-transplant-rejection/. Accessed February 18, 2026.« Back to 2020 American Transplant Congress