TRANSFORM Study (NCT01950819): Application of the Ibox Clinical Trial Simulation Tool to Project Long-Term Kidney Allograft Outcome
1INSERM U970, Paris Transplant Group, Paris, France, 2Department of Nephrology, Hospital del Mar/Universitat Autònoma de Barcelona, Barcelona, Spain, 3Department of Surgery/Kidney Transplant Service, University of California, San Francisco, CA, 4Research and Development, Novartis Pharma AG, Basel, Switzerland, 5TRANSFORM Investigators, Basel, Switzerland
Meeting: 2020 American Transplant Congress
Abstract number: 223
Keywords: Graft survival, Kidney transplantation, Multicenter studies, Outcome
Session Information
Session Name: Novel Tools to Assess Immunosuppressive Efficacy
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
*Purpose: The development of pharmaceutical agents in transplantation is currently limited by long waits for hard endpoints. We sought to use a risk stratification system in a large randomized control trial (RCT) and determine individual patient long-term graft survival and eGFR decline.
*Methods: We used validated data from the TRANSFORM trial (NCT01950819), a RCT that compares kidney transplant recipient to receive everolimus with reduced-exposure calcineurin inhibitor (CNI) or mycophenolic acid (MPA) with standard-exposure CNI. We applied the iBox system (NCT03474003), an integrative and validated risk score which used the parameters measured at 1 year after randomization (primary end point time line) and projected patient’s individual long-term allograft survival.
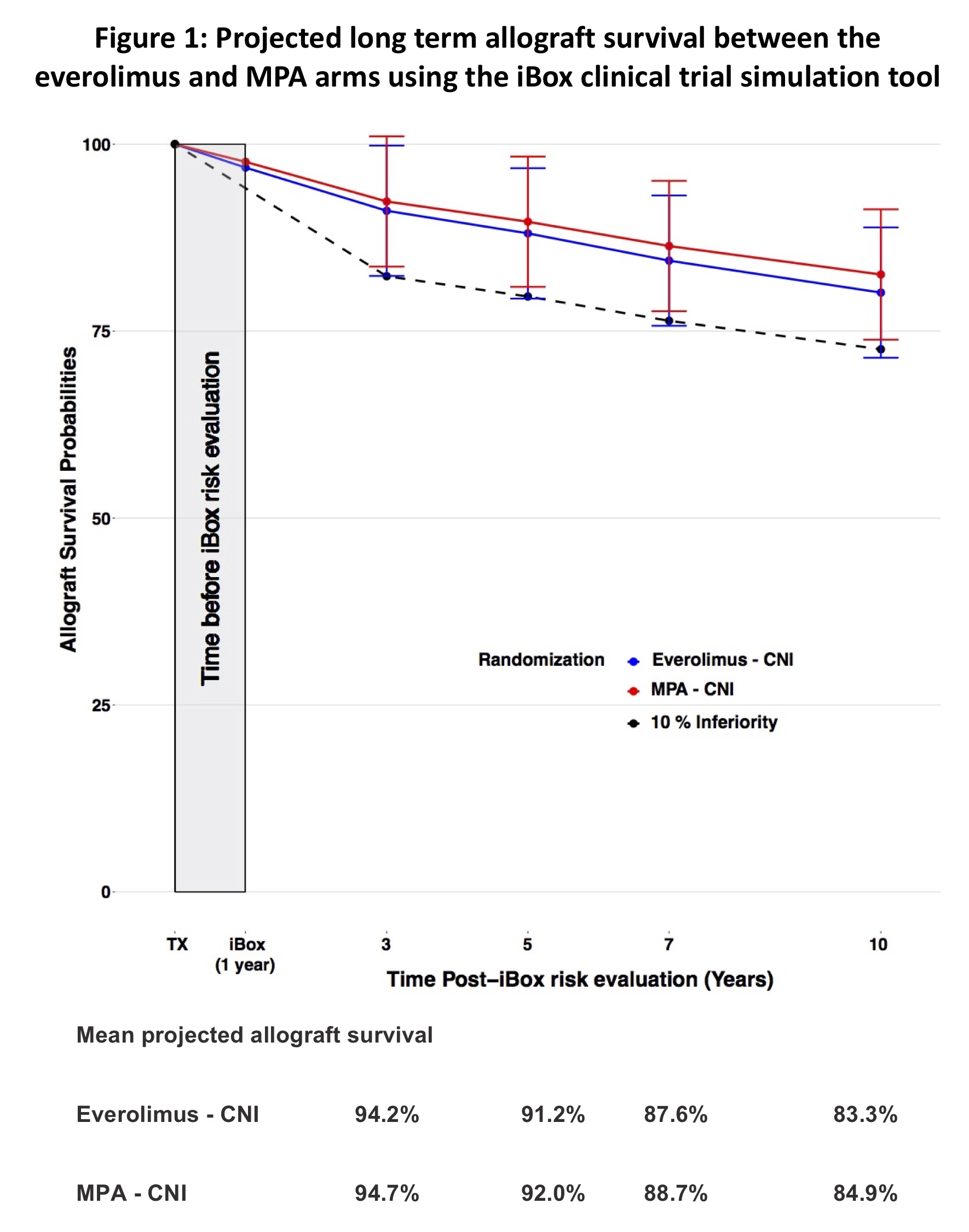
*Results: A total of 1872 patients (940 in the everolimus arm and 932 in the MPA arm) reached the 1 year after transplant primary endpoint. Mean estimated glomerular filtration rate was 55.5±19.9 mL/min/1.73m2 in the everolimus arm vs 56.1±19.0 in the MPA arm. The mean protein/creatinin ratio was 0.33±0.68 g/g in the everolimus arm vs 0.25±0.62 in the MPA arm. The incidence of active-ABMR and acute-TCMR of 7.1% and 7.2% in the everolimus arm vs 6% and 7.1% in the MPA arm. The rate of circulating anti-HLA DSA was 13.8% in the everolimus arm vs 16.1% in the MPA arm. These immunological, functional and histological parameters were entered into the iBox risk prediction system, which translated to an overall patient graft survival at 3, 5 and 10 years after randomization of 94.2 vs 94.7% %, 91.2% vs 92.0% and 83.3% vs 84.9% in the everolimus and MPA arms respectively (95%CI -3.1% to 0.2%, below the non-inferiority margin of 10%) Figure 1.
*Conclusions: The iBox system confirms the non-inferiority of everolimus vs MPA 10 years after patient’s randomization in the RCT. Given the unmet need for surrogate end point for clinical trials, this study shows the potential of a clinical trial simulation tool to fast track the development and approval of pharmaceutical agents.
To cite this abstract in AMA style:
Divard G, Aubert O, Raynaud M, Pascual J, Vincenti F, Bernhardt P, Legendre C, Loupy A. TRANSFORM Study (NCT01950819): Application of the Ibox Clinical Trial Simulation Tool to Project Long-Term Kidney Allograft Outcome [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/transform-study-nct01950819-application-of-the-ibox-clinical-trial-simulation-tool-to-project-long-term-kidney-allograft-outcome/. Accessed February 3, 2026.« Back to 2020 American Transplant Congress