An Optimized Integrative Model Using Urinary Chemokines for Noninvasive Diagnosis of Acute Allograft Rejection
1Nephrology and Kidney Transplantation, Inserm U1151, Necker Hospital, Paris, France, 2Nephrology and Kidney Transplantation, Strasbourg University Hospital, Strasbourg, France, 3Pathology, Necker Hospital, Paris, France
Meeting: 2020 American Transplant Congress
Abstract number: 90
Keywords: Kidney transplantation, Multivariate analysis, Non-invasive diagnosis, Polyma virus
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes I
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: The aim of this study was to optimize the performance of urinary chemokines C-X-C motif ligand (CXCL) 9 and CXCL10 as noninvasive diagnostic markers of acute rejection (AR) in kidney transplant recipients (KTRs).
*Methods: CXCL9 and CXCL10 were quantified in 391 urine samples concomitant with graft biopsy, urinalysis and blood BK virus (BKV) viral load assessment. Confounding factors for chemokine elevations were identified and integrated to build a multiparametric noninvasive model of AR diagnosis.
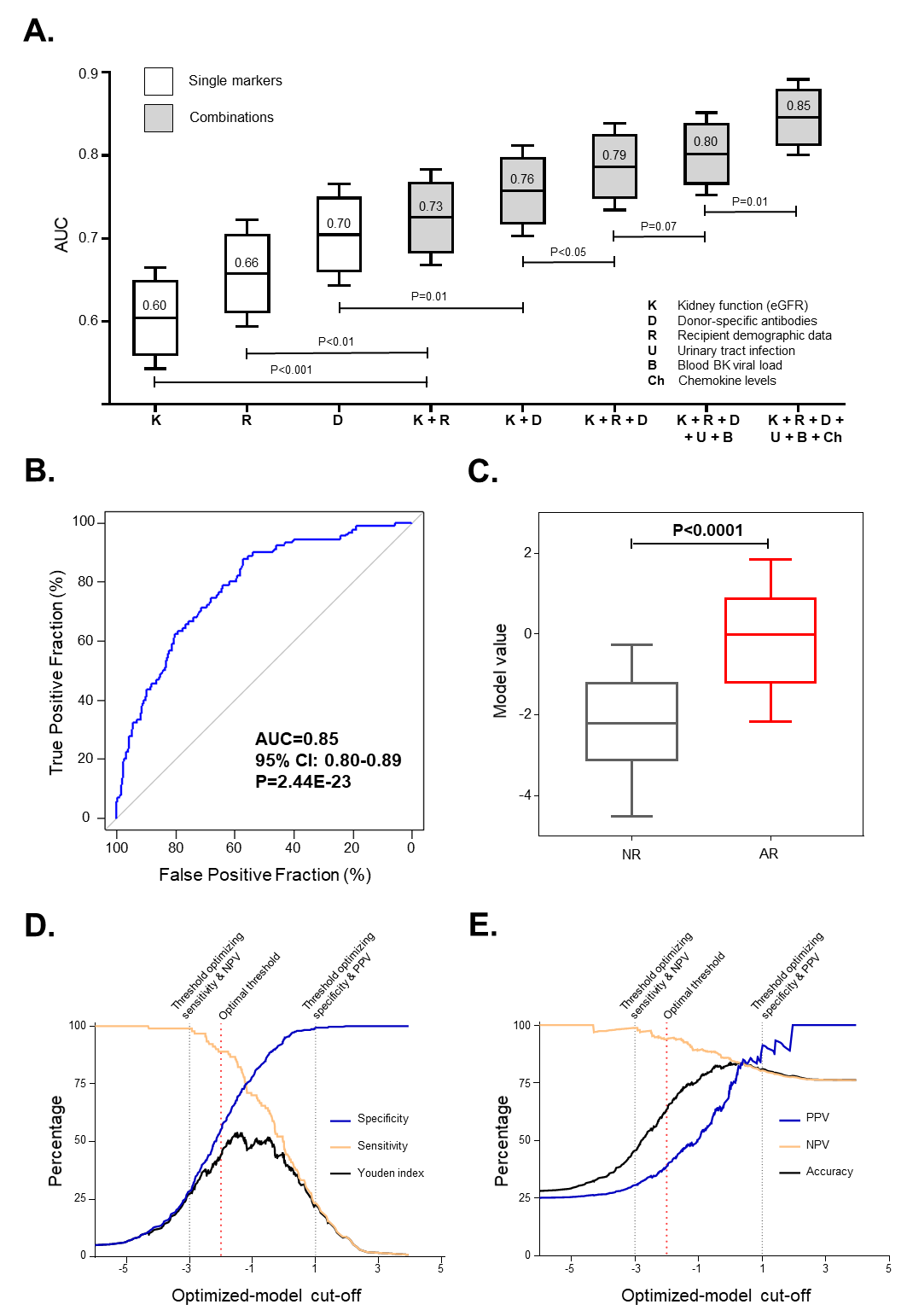
*Results: Both urinary tract infection (UTI) and BKV reactivation were associated with increases in CXCL9 and CXCL10 levels. Compared to nonviremic patients, BKV-viremic KTRs had similarly increased levels of CXCL9 and CXCL10 in both the absence (P<0.001 and P<0.0001, respectively) and presence (P<0.01 and P<0.0001) of BKV-associated nephropathy (BKVAN). Next we built several multivariate regression models for the diagnosis of AR. Panel A illustrates the incremental performances of various biomarkers used alone or in combinations. An optimized 8-parameter model (including recipient age and sex, estimated glomerular filtration rate, donor-specific antibodies, UTI, BKV blood viral load, and urinary CXCL9 and CXCL10) diagnosed AR with high accuracy (area under the curve [AUC]=0.85, 95% confidence interval [95% CI] 0.80-0.89; P<0.0001) in an unselected cohort (Panel B-C). These 8 parameters diagnosed AR with an AUC of 0.92 (P<0.0001) in an external validation cohort. This model remained highly accurate in cases of stable graft function (AUC=0.80) or at the time of graft dysfunction (AUC=0.85) and within the first year (AUC=0.86) or later (AUC=0.90). A model threshold optimizing sensitivity (Panel D-E) gives a NPV of 0.99, while optimizing specificity gives a PPV of 0.88. The optimal threshold for clinic of -2 avoids 157 unnecessary biopsies while missing 10% of AR.
*Conclusions: Considering confounding factors rather than excluding them, we optimized a multiparametric diagnostic model for AR of kidney allografts, which achieves unprecedented accuracy for a noninvasive diagnostic tool.
To cite this abstract in AMA style:
Tinel C, Devresse A, Vermorel A, Marx D, Caillard S, Legendre C, Rabant M, Anglicheau D. An Optimized Integrative Model Using Urinary Chemokines for Noninvasive Diagnosis of Acute Allograft Rejection [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/an-optimized-integrative-model-using-urinary-chemokines-for-noninvasive-diagnosis-of-acute-allograft-rejection/. Accessed February 5, 2026.« Back to 2020 American Transplant Congress