The Impact of Thy1 Expression of Fibroblast for Chronic Lung Allograft Dysfunction
1Surgery, University of Virginia, Charlottesville, VA, 2Biomedical Engineering, University of Virginia, Charlottesville, VA
Meeting: 2020 American Transplant Congress
Abstract number: 83
Keywords: Fibrosis, Lung transplantation, Rejection
Session Information
Session Name: From Bench to Community to Bedside in Lung Transplantation
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
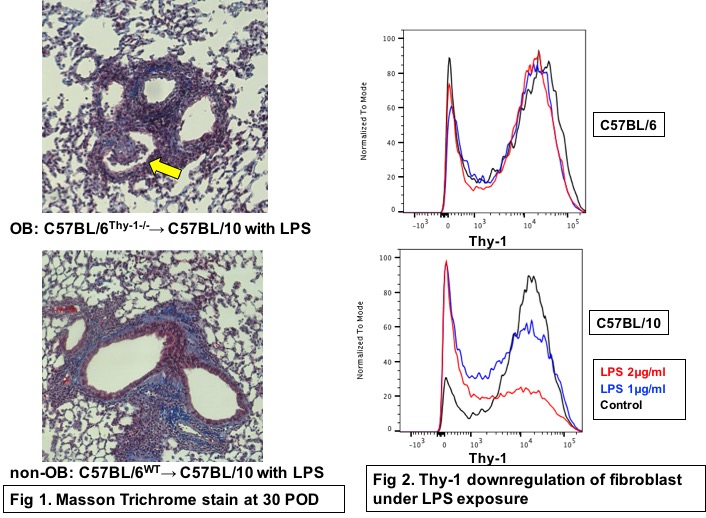
*Purpose: Chronic lung allograft dysfunction (CLAD) result from excessive immune activation and excessive collagen deposition by fibroblasts within the lung parenchyma or airway.Bronchiolitis obliterans (OB) is a specific form of CLAD that results in the growth of fibrotic lesions in the airway. Thy-1 is a glycoprotein demonstrated to play a role in downregulating fibroblast differentiation to myofibroblasts. The role of Thy-1 in lung transplant-related CLAD, however, has not been explored. We find this somewhat surprising since multiple immunologic and non-immune insults have been described to contribute to the final common pathway of collagen deposition by fibroblasts in CLAD.
*Methods: To investigate the role of stromal-cell specific expression of Thy-1 in OB, we utilized the minor antigen mismatched C57BL/6 (B6)→C57BL/10 (B10) model of murine lung transplantation (LTx). Specifically we transplanted either wild type B6 or Thy-1 deficient grafts to B10 recipient mice (B6wt→B10 vs. B6Thy1-/-→B10). To mimic bacterial infection some mice received 6 doses of LPS (5μg in 50μl PBS/dose) intratracheally every 4 days until sacrifice on postoperative day 30 while some mice were treated with saline. Grafts were assessed histologically after sacrifice as having OB or not and immunohistochemical (IHC) staining for Thy-1 was performed upon sacrifice. To assess the role of LPS on fibroblast-specific Thy-1 expression, we utilized primary cell cultures of murine lung fibroblasts isolated from both B6 and B10 mice, using media containing varying concentrations (0μl, 1μl or 2μl/ml) of LPS.
*Results: No OB was evident in either B6wtor B6Thy1-/-→B10 lung transplants treated with saline while 3/12(25%) of B6wt→B10 and 4/11(36%) of B6Thy1-/-→B10 mice treated with LPS developed OB (Fig 1). IHC showed most of OB and fibrotic lesion in WT group consisted of Thy-1 negative fibroblast. In vitro LPS significantly downregulated Thy-1 in fibroblast(P<0.05) (Fig 2).
*Conclusions: Here we demonstrate the link between the downregulation of Thy-1 and CLAD. While the link between bacterial infection and CLAD has been postulated to involve activation of the immune system here we demonstrate that LPS-mediated loss of Thy-1 on stromal cells similarly results in proliferative airway lessions. Our findings extent our understanding of chonic rejection and suggest that Thy-1 upregulation may serve as a therapeutic option to prevent chronic rejection.
To cite this abstract in AMA style:
Hata A, Guo Y, Li D, Mei Z, Manafi A, Banerjee A, Mahgoub B, Barker TH, Krupnick AS. The Impact of Thy1 Expression of Fibroblast for Chronic Lung Allograft Dysfunction [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-impact-of-thy1-expression-of-fibroblast-for-chronic-lung-allograft-dysfunction/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress