Multicenter Experience Evaluating Outcomes of HCV-Seropositive Donors to HCV-Seronegative Recipients Liver Transplantation
K. Wijarnpreecha1, B. Aqel2, S. Pungpapong1, C. Taner3, K. Reddy4, M. Leise5, R. Dickson2
1Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, FL, 2Gastroenterology and Hepatology, Mayo Clinic, Scottsdale, AZ, 3Transplant, Mayo Clinic, Jacksonville, FL, 4Transplant and Hepatobiliary Surgery, Mayo Clinic, Scottsdale, AZ, 5Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN
Meeting: 2019 American Transplant Congress
Abstract number: D234
Keywords: Hepatitis C, Liver, Liver transplantation, Outcome
Session Information
Session Name: Poster Session D: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Grafts from hepatitis C virus (HCV)-seropositive donors can now be considered for liver transplant (LT) due to the advent of direct-acting antiviral agents (DAA). We report our multicenter experience evaluating the use of HCV-seropositive donors to HCV-seronegative recipients LT.
*Methods: This is a retrospective analysis of prospectively collected database of an ongoing multicenter IRB-approved research study expanded into clinical protocol to evaluate adult HCV-seronegative LT recipients who received grafts from HCV-seropositive donors.
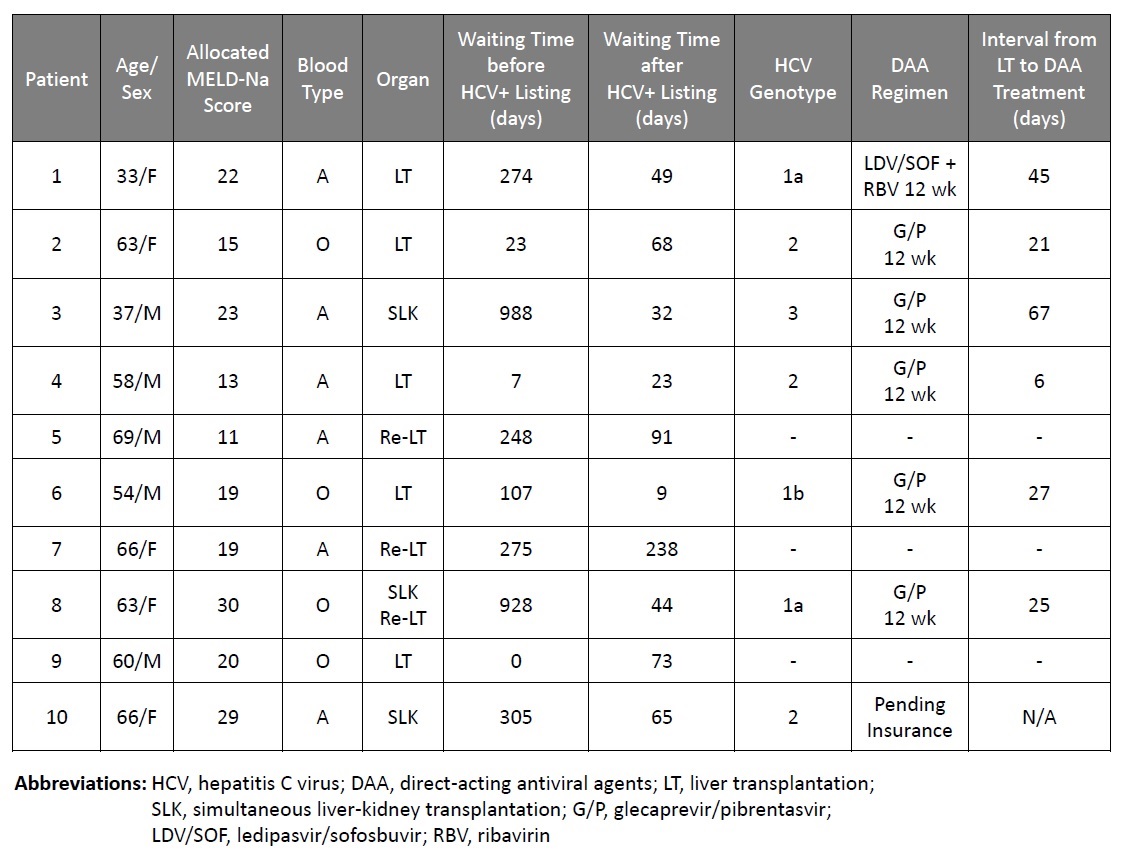
*Results: Since January 2018, 10 HCV-seronegative LT recipients received grafts from HCV-seropositive brain-dead donors [3 with negative nucleic acid testing (NAT) and 7 with positive NAT] with the median allocated MELD-Na score of 20. Two underwent simultaneous liver-kidney transplant (SLK), 2 repeat LT and 1 with SLT/repeat LT. The median waiting times were 266 days (range: 0-988) prior to listing for HCV-seropositive donor and 57 days (range: 9-238) to LT after listing for HCV-seropositive donor. No recipient of an HCV-NAT negative graft developed HCV viremia with median post LT follow-up of 48 days. All 7 patients who received HCV-NAT positive grafts had HCV viremia confirmed within 5 days after LT with the median peak HCV RNA of 33.1 million IU/mL (range 1-97.5 million). DAA treatment was started at the median time of 32 days (range 6-62) after LT: glecaprevir/pibrentasvir (G/P) for 12 weeks (5 patients), ledipasvir/sofosbuvir (LDV/SOF) + ribavirin (RBV) for 12 weeks (1 patient), and 1 patient is waiting for insurance approval. All were HCV RNA undetectable by week 4. Mild AST/ALT elevation was observed, but not associated with graft dysfunction. Acute cellular rejection was biopsy diagnosed in 3 patients, acute membranous nephropathy due to HCV infection in one patient. All were NAT positive primary LT patients. No graft loss or death was observed.
*Conclusions: LT using HCV-seropositive grafts to HCV-seronegative recipients resulted in acceptable short-term outcomes in our multicenter experience. This preliminary data supports continued use of HCV-seropositive grafts with expansion to SLK or repeat LT. Careful ongoing assessment regarding complications and timing of treatment is required.
To cite this abstract in AMA style:
Wijarnpreecha K, Aqel B, Pungpapong S, Taner C, Reddy K, Leise M, Dickson R. Multicenter Experience Evaluating Outcomes of HCV-Seropositive Donors to HCV-Seronegative Recipients Liver Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/multicenter-experience-evaluating-outcomes-of-hcv-seropositive-donors-to-hcv-seronegative-recipients-liver-transplantation/. Accessed February 21, 2026.« Back to 2019 American Transplant Congress